Biology Reference
In-Depth Information
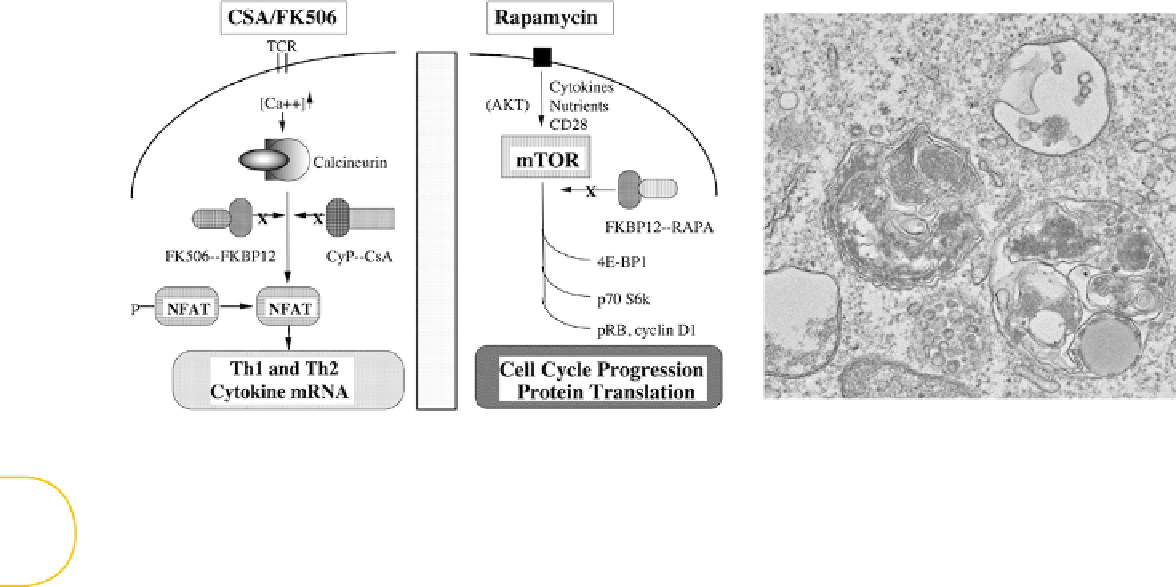
FIGURE 11.2
Use of
ex vivo
rapamycin to modulate Th1/Th2 biology. (Left) Rapamycin (Sirolimus) modulates T-cell biology through mTOR inhibition rather than calcineurin inhibi-
tion, which is the mechanism of action for the other commonly utilized immune suppression drugs in transplantation, cyclosporin A and FK506 (Tacrolimus). mTOR inhibi-
tion blocks multiple T-cell surface receptor signaling pathways, thereby decreasing T-cell protein translation (4EBP1 pathway), protein phosphorylation (p70S6K pathway),
and cell cycle progression; by comparison, calcineurin inhibition primarily inhibits T cells at the mRNA transcriptional level. (Right) mTOR in the activated state promotes
cell growth by inhibiting autophagy, whereas mTOR inhibition by rapamycin results in T-cell autophagy (as shown in the electron micrograph). Rapamycin-resistant (RR),
postautophagy T cells possess an antiapoptotic T-cell phenotype and polarized T cells of each type mediate increased
in vivo
effects upon adoptive transfer (RR-Th1/Tc1
cells mediate increased GVHD, whereas RR-Th2/Tc2 cells are enriched in their capacity to prevent GVHD).
232
shifted post-transplant immunity to a Th2/Tc2 phenotype
[61]
and that
human CD8
+
T cells could be rendered rapamycin resistant (RR)
[77]
.
Contrary to our initial expectations, we found that it was possible to polarize
toward either a Th1/Tc1 or a Th2/Tc2 phenotype in the presence of a supra-
pharmacologic concentration of rapamycin depending on whether IL-12 or
IL-4 was utilized during optimal, APC-free costimulation
[78]
. Importantly,
in marked contrast to initial reports that identified rapamycin as an agent
that induced T-cell tolerance even in the presence of costimulation
[79]
,
we found that RR-Th1/Tc1 cells were enriched in their capacity to induce
GVHD, whereas RR-Th2/Tc2 cells were increased in their capacity to cross-
regulate Th1/Tc1 responses
[78]
. As such, we reasoned that the increased
therapeutic efficacy of adoptively transferred T cells of Th1 or Th2 pheno-
type was attributable to a more general functional characteristic induced
by rapamycin; to address this, we evaluated whether modulation of T-cell
differentiation status or apoptotic threshold might be operative.
In murine models, autologous T cells with more limited differentiation sta-
tus were associated with improved anti-tumor effects upon adoptive trans-
fer: that is, transfer of naïve T cells was more potent than that of T
CM
cells,
which were themselves more potent than terminally differentiated T
EM
cells
[80]
. These findings were at first somewhat counterintuitive because
T
EM
cells displayed enhanced IFN-γ secretion and more potent cytolytic
capacity prior to adoptive transfer; however, less differentiated T cells were
able to sustain engraftment post-transfer, with the net result of heightened
effector function
in vivo.
In our murine studies involving allogeneic RR-Th2
cells, which express a T
CM
phenotype, we found that the
in vivo
capacity to
produce type II cytokines was increased by approximately 10-fold relative
Search WWH ::

Custom Search