Biology Reference
In-Depth Information
(A)
(B)
144
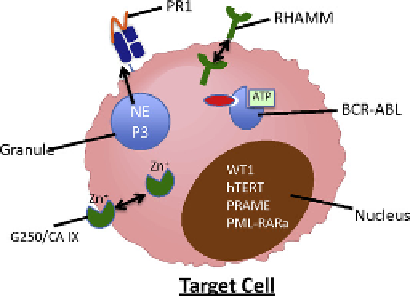
FIGURE 7.1
Tumor-associated antigens (TAAs). (A) TAAs originate in various cellular compartments, including the
nucleus, granules, cell membrane and cytoplasm. However, in
order for effective antigen presentation to occur, (B) the TAA must localize to cellular compartments that facilitate antigen presentation. Cytoplasmic proteins are readily
available for antigen presentation. Specifically, cytoplasmic proteins can be ubiquitinated, degraded by proteasome and subsequently presented on MHC class I. The
abnormal expression of TAA by malignant cells, either through overexpression or aberrant localization, can lead to preferential presentation of TAA-derived immunogenic
epitopes on the target cell surface. The expression of these small (9-14 amino acids) peptides is sufficient for recognition by a primed immune cell.
immunotherapeutic approaches, including allo-HSCT. Because the immu-
nogenicity of such proteins is dependent on small amino acid sequences
(9-14 amino acids) derived from that protein that are subsequently pre-
sented on MHC class I or II, the amino acid mutation(s) may not be con-
tained within the portion of the protein that is processed and from which
the immunogenic epitope is derived. Unless the immunogenic epitopes that
are presented on cell-surface MHC encompass the amino acid mutation(s),
cells that harbor mutated proteins will not be distinguished from normal
counterparts purely based on presence of the amino acid mutation.
However, other distinct features of malignant cells such as aberrant expres-
sion or mutations in regulatory proteins can influence the timing, degree
of expression and subcellular localization of normal cellular proteins.
The aberrant expression of normal cellular proteins in malignant cells,
which are naturally shielded from the MHC-processing machinery, can
subsequently render them immunogenic (i.e. aberrantly expressed self-
antigens). This is exemplified by the primary azurophil granule proteases
(PGP) neutrophil elastase (NE), proteinase 3 (P3) and cathepsin G (CG),
which have been successfully targeted by immunotherapies in hemato-
poietic malignancies. Although no mutations have been reported in these
proteases in leukemia, these PGP were shown to be highly expressed by
myeloid leukemia cells outside azurophil granules where they are nor-
mally found
[1]
. The aberrant expression of these unmutated self-antigens
is believed to facilitate the presentation of immunogenic epitopes that are
derived from these antigens.


Search WWH ::

Custom Search