Biology Reference
In-Depth Information
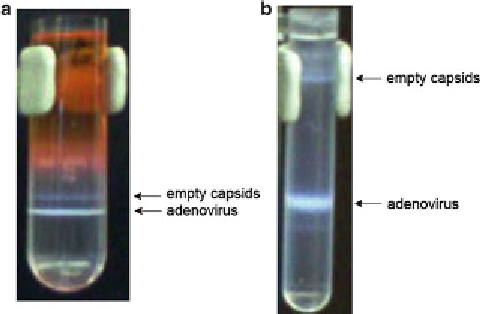
Fig. 3
Visualization of adenovirus and empty capsids after the initial step gradient
(
a
) and after the second isopycnic gradient (
b
), respectively
1. Transfer the recovered vector band from the previous step into
a new polyallomer centrifuge tube for SW40 Ti rotor.
3.3.2 Second Isopycnic
Gradient
2. In a second tube add CsCl (1.34 g/mL) to balance the rotor.
Balance the tubes and load in rotor.
3. Centrifuge for 22 h at 155,000 ×
g
18 °C in a Beckman SW40
rotor, maximum brake.
4. Remove tubes from rotor with forceps.
5. The vector appears as an opaque band near the center of the
tube (
see
Fig.
3b
). Remove band as described above in a maxi-
mum of 2.5 mL.
1. Prepare a PD-10 column following the manufacturer's instruc-
tions. Load up to 2.5 mL of purifi ed adenovirus on the
column.
2. Label 1.5 mL tubes. Start to collect eluted fractions by adding
0.5 mL of PBS 1× Ca
2+
/Mg
2+
. Repeat this seven times.
The adenovirus is clearly visible as an opaque solution between
fractions 4 and 7.
3. Add sterile glycerol to the fractions to a fi nal concentration
of 10 %.
4. Titrate fractions 3-8 using anti-Ad/hexon (
see
Subheading
3.4.1
).
5. Select and pool the desired fractions.
6. Aliquot in small volumes in 0.5 mL tubes and store at −80 °C
as quickly as possible (
see
Note 11
).
3.3.3 Desalting
Column and Storage