Biology Reference
In-Depth Information
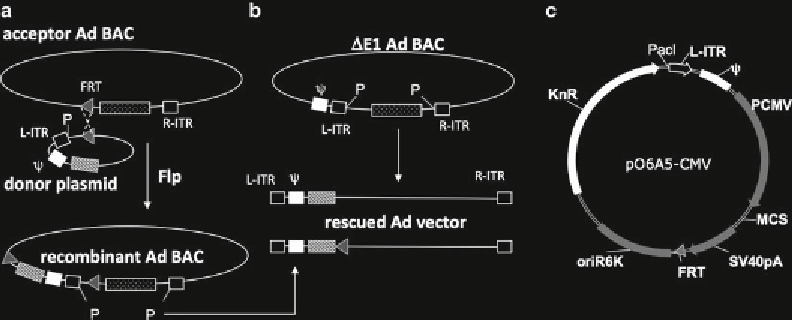
Fig. 2
(a)
Schematic representation of the Flp/FRT recombination for construction of fi rst generation Ad vec-
tors. This approach is based on Flp-mediated unifi cation (recombination of two components: An acceptor
Ad-BAC and a donor plasmid. The acceptor Ad-BAC carries the full Ad genome except its very left terminus
including the left ITR (L-IRT) the packaging signal (
) and the E1 region (not shown). Instead, in this construct
the left terminal sequences are replaced by a FRT site. The donor plasmid which possesses a complementary
FRT site carries the left terminal Ad sequences suffi cient to reconstitute a fi rst generation Ad vector (L-ITR and
packaging signal) is fused to a transcription unit for the gene of interest (
hatched box
). After Flp recombination
the donor plasmid is entirely inserted into the acceptor BAC. (
b
) Activation of a traditionally designed E1
deleted Ad-BAC vector: the linear Ad sequence is released by digestion of
Pac
I restriction endonuclease at ITR
adjacent
Pac
I sites (P). A similar Ad genome can be released from the recombinant Ad-BACs generated by the
Flp approach after the same treatment. The only difference is a single FRT site between the transgene and the
rest of the vector genome. Other donor plasmid sequences are left behind connected to the BAC vector
sequences (
dotted box
in part A). (
c
) Map of the cloning vector pO6A5-CMV used for construction of the donor
plasmids for gene expression. The gene of interest can be inserted into the multiple cloning site (MCS)
Ψ
with the DH10B cells carrying pBA5-FRT and pCP20.
Incubate the culture overnight at 32 °C.
6. Add 1 mL of overnight culture into two culture fl asks with
200 mL LB containing 25
μ
g/mL chloramphenicol and
g/mL ampicillin and continue the incubation at 32 °C in
a shaking incubator until they reach density of OD
600 nm
of
0.55-0.6 (takes approximately 3 h).
7. Cool the induced culture down immediately by transferring
the fl ask directly to the ice pocket and keep them on ice for
further 15 min. Transfer the culture to four 50 mL Falcon
tube.
8. Centrifuge the cells for 5 min at 7,000 ×
g
on +1 °C. Remove
all supernatant and resuspend the bacterial pellet well in 1 mL
ice-cold sterile 10 % glycerol by pipetting up and down. Collect
the four suspensions in one 50 mL Falcon tube and add 45 mL
ice-cold sterile 10 % glycerol.
9. Repeat
step 8
twice. The bacterial pellet will get loose, be care-
ful to keep them in the tube while removing the supernatants
after centrifugations.
50
μ