Biology Reference
In-Depth Information
microdomains; however, it remains to be verified given the uncertain status of
EGFR as an HCMV entry receptor (Wang et al. 2005).
Envelope and Membrane Fusion
The exact mechanism of the fusion step is not clear. Over the past several years, it
has become increasingly apparent that amino acid heptad repeat (HR) motifs,
which encode alpha-helical coiled-coils, are playing a role in the fusion process of
HCMV. These coils are very well characterized in more simple viral fusogenic sys-
tems, such as with influenza, HIV, and Ebola viruses, but they are only beginning
to be understood in the more complex herpesviruses (Weissenhorn et al. 1999). It
is known that synthetic peptides from the HR region of HCMV gB and gH and β
amino acid oligomers derived from the HR region of gB specifically inhibit HCMV
entry (Lopper and Compton 2004; English et al. 2006). Additionally, the HR region
of gB appears to be important because mutation of hydrophobic amino acid resi-
dues in this region results in a replication-deficient virus (M.K. Isaacson and
T. Compton, unpublished results).
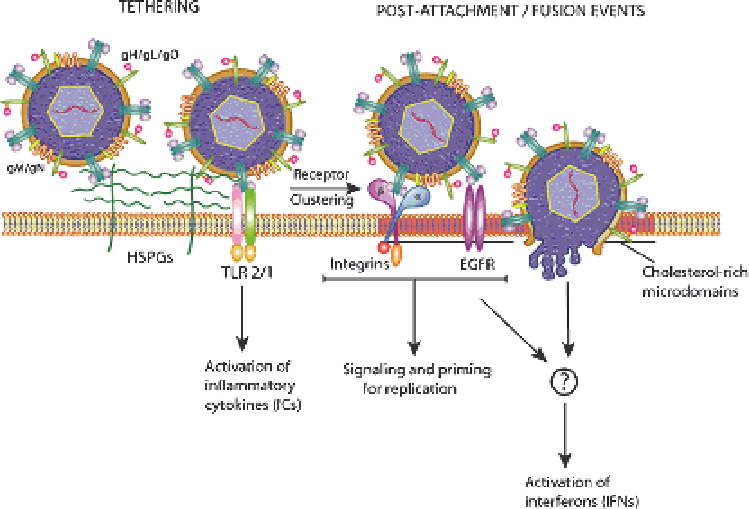
Our current model for HCMV entry (Fig. 1) consists of an initial tethering step
to HSPGs on the cell surface mediated by gB and the gM/gN complex (Kari and
Gehrz 1992, 1993; Compton et al. 1993). The virus then quickly moves to a more
stable binding step, most likely mediated by gB given its biphasic binding properties
Fig. 1
HCMV entry model