Biology Reference
In-Depth Information
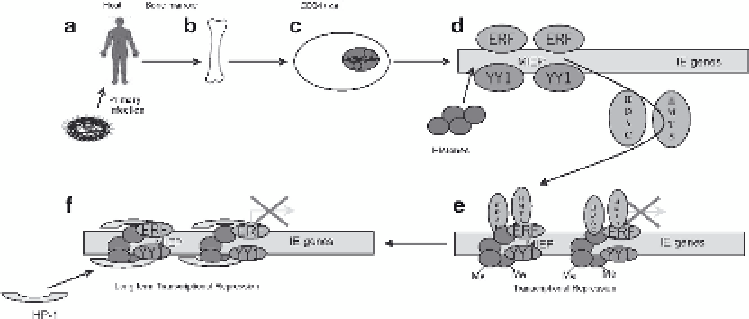
Fig. 2
The establishment of HCMV latency is promoted by chromatin structure. Following infection
(
a
), HCMV infects the cells of the bone marrow (
b
) and establishes a latent infection of the CD34
+
cells resident therein (
c
). High levels of cellular transcriptional repressors such as ERF and YY1
(
d
) repress the MIEP. As well as transcription factor binding, histone proteins are recruited to the
MIEP which become targets for histone deacetylases and histone methyltransferases that are
recruited by YY1 and ERF (
e
). These methylated histones become targets for the recruitment of
HP-1, which augments repression and the establishment of latency (
f
). Whether any viral products
expressed during latency that are important for the repression of the MIEP in this model is, to
date, unknown
To date, there is a good consensus that HCMV infects CD34
+
haematopoietic
stem cells and establishes a latent infection in them. Whilst this is demonstrably
true, it has also been suggested that subsets of CD34
+
cells may show susceptibility
for HCMV productive infection. A study by Goodrum et al. (2004) that analysed
differential outcomes of HCMV infection in sorted populations of haematopoietic
CD34
+
stem cells concluded that infection of one subset of CD34
+
cells (CD34
+
but CD38
-
) established the hallmarks of a latent infection (Goodrum et al. 2004),
i.e. no detectable virus production but the ability to reactivate upon cellular differ-
entiation. In contrast, other CD34
+
cell subpopulations were fully productive for
HCMV infection, whilst more mature CD34
+
stem cell subpopulations appeared to
undergo abortive infection and failed to maintain latent viral genomes. This sug-
gests that the outcome of infection of different CD34
+
stem cell subpopulations
could depend on the exact phenotype of each stem cell subpopulation. Indeed, there
is increasing evidence of early lineage commitment in the haematopoietic stem cell
compartment such that a dendritic cell fate, although not irreversible (O'Garra and
Trinchieri 2004), is thought to be determined at earlier stages of progenitor cell
development (Olweus et al. 1997; Monji et al. 2002). Taken together, these observa-
tions could support the hypothesis that HCMV infection of CD34
+
stem cells,
resulting in latent viral carriage, is restricted to certain subpopulations of CD34
+
stem cells. These cells are restricted to specific myeloid cell fates and this mecha-
nism may explain why the carriage of HCMV genomes occurs in some but not all
cell types of the myeloid lineage.