Biology Reference
In-Depth Information
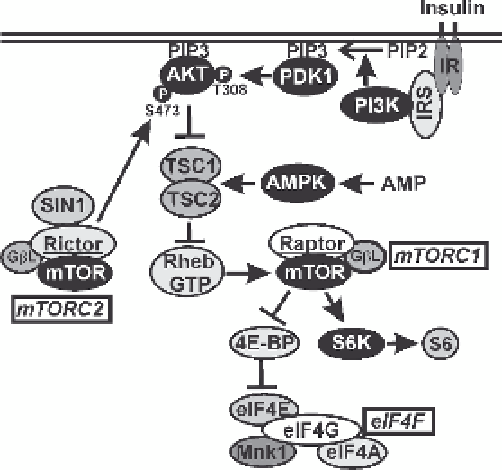
Fig. 1
The PI3K-Akt-mTOR signaling pathway with emphasis on the control of cap-dependant
translation via eIF4F. Details are discussed in the text
The link between Akt and mTORC1 is (1) the tuberous sclerosis complex (TSC;
reviewed in Luo et al. 2005), made up of TSC1 [hamartin] and TSC2 [tuberin] and (2)
Rheb-GTP, a member of the Ras superfamily which binds the N-terminal lobe of
the mTOR kinases catalytic domain, allowing mTOR activation (Astrinidis and
Henske 2005; Long et al. 2005a, 2005b). Regulation of Rheb-GTP levels is medi-
ated by the GTPase-activating function of the TSC, which stimulates the intrinsic
GTPase activity of Rheb, converting it from Rheb-GTP to Rheb-GDP, the inactive
form that cannot activate mTORC1. Thus Akt's phosphorylation of the TSC inac-
tivates it, allowing Rheb-GTP levels to remain high in order to activate mTORC1.
Background: The Complexes of mTOR Kinase
and Their Activities
mTOR kinase is found in two complexes that differ in their major binding partner
(Fig. 1): raptor (
r
egulatory
a
ssociated
p
rotein of
TOR
) in mTORC1 and rictor
(
r
apamycin-
i
nsensitive
com
panion of m
TOR
) in mTORC2 (Kim et al. 2002;
Sarbassov et al. 2004). Both complexes contain a small protein called GβL that binds
to the kinase domain of mTOR kinase and stabilizes the interaction with raptor and
rictor (Kim et al. 2003). An additional protein, SIN1, found in mTORC2, maintains
the integrity of the complex and regulates activity and substrate specificity (Jacinto
et al. 2006; Polak and Hall 2006; Yang et al. 2006a). It is important to note that under
normal conditions the two complexes differ in their sensitivity to the drug rapamycin;
mTORC1 is sensitive and mTORC2 is insensitive (Sarbassov et al. 2004).