Agriculture Reference
In-Depth Information
Box 3.1
Formation and Properties of Domains
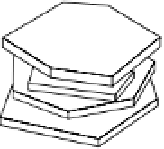
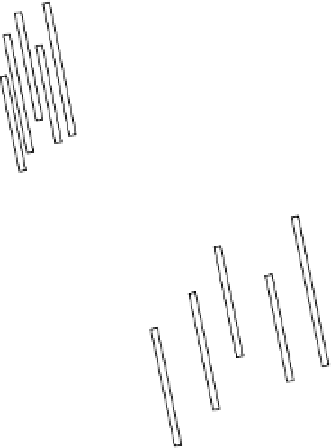
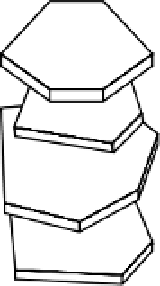
Electron micrographs of thin sections of soil show crystals of montmorillonite,
illite, or vermiculite stacked roughly parallel in the direction of the
c
axis (see fig.
B2.4.1), to form clay domains. Flocculation of the clay crystals in a
face-to-face
array is necessary for domain formation. This kind of flocculation is favored when
exchangeable cations of high charge (Al
3
or Ca
2
) are present, and the total salt
concentration in the soil solution is high. For example, when Ca
2
is the
dominant exchangeable cation, the basal spacing of the clay is at a minimum of
1.4 nm in a soil solution as concentrated as M CaCl
2
. Spacing increases to no
more than 1.9 nm as the solution concentration decreases to that of distilled water
(fig. B3.1.1). Thus, each Ca-clay domain is a stable entity in water, showing
limited swelling, because the expansion of the
diffuse double layer
(
DDL
) at the
crystal surfaces is suppressed (section 4.5.2). The domains are only likely to be
disrupted if more than about 15% of the moles of charge of Ca
2
are replaced by
Na
. Na
ions induce much greater swelling because these ions are less strongly
attracted to the surface than Ca
2
ions, and the osmotic intake of water into the
DDL
is greater. If the Na-Ca clay domain is placed in distilled water, excessive
swelling may lead to clay deflocculation (section 3.2.3).
In more highly weathered soils, where 1:1 clays such as kaolinite predominate,
clay crystals are attracted in
edge-to-face
flocculation to form a “cardhouse” type of
Figure B3.1.1
Face-to-face flocculation with 2:1 clay mineral crystals and their expansion on diluting
the soil solution (redrawn from White 1997).
Ca-montmorillonite domains,
concentrated solution
Dilution of
solution
Limited
swelling
Crystals in the domains
move farther apart, but
domains remain intact
(continued)