Agriculture Reference
In-Depth Information
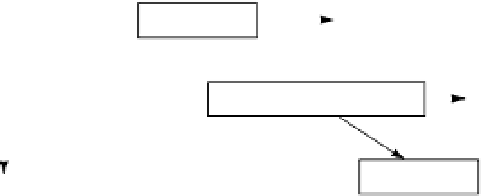
“Cascade effect” in decomposition
fresh residues
CO
2
k
1
biomass
CO
2
k
2
decreasing
C:N
stabilized OM (humus)
CO
2
ratio
The “cascade effect” in
organic matter
decomposition.
Figure 2.17
k
3
inert OM
first-order rate constant. When fresh residues are added to the soil, there is a “cas-
cade effect” as the most easily decomposed parts are attacked first and progres-
sively more resistant parts accumulate. Virtually all the C passes through the mi-
crobial biomass, which in turn dies and decomposes, adding to the more resistant
remains. The process is illustrated in figure 2.17. The “stabilized” organic matter
may consist of chemically resistant compounds, as in humus, or be physically pro-
tected, because of adsorption or its location in very small pores (see below). In the
field, there is a wide spectrum of
k
values for different C substrates, as expressed
by their half-lives
t
0.5
, where
0.693
t
0.5
(2.2)
k
These half-lives range from
1 month to
30 years, with the half-life for
inert organic matter exceeding 50,000 years. However, for practical purposes, we
can use average values of
k
in equation 2.1 to model the rate of
SOM
decompo-
sition in individual soils.
Soil Properties and Environmental Factors
Soil moisture, O
2
supply, pH, and temperature affect the rate of organic matter
decomposition. The first two factors tend to counteract one another because when
soil moisture is high, a deficiency of O
2
may restrict decomposition. However,
when the soil is dry, moisture but not O
2
will be the limiting factor. pH has lit-
tle effect, except below 4 when the decomposition rate slows, as in the case of mor
humus or cold, wet peaty soils. On the other hand, temperature has a marked ef-
fect, not only on plant growth and hence litter return, but also on litter decom-
position through its effect on the rate of microbial respiration. For example, or-
ganic residues in soils of humid, hot regions (mean temperature 26°C) can
decompose up to four times faster than in soils of humid, temperate regions (mean
temperature 9°C).
Adsorption of C compounds by clays and sesquioxides generally slows down
their rate of decomposition. For example, the accumulation of organic matter in
soils derived from volcanic ash is attributed to the stabilization of HA and FA
fractions through their adsorption on the clay minerals imogolite and allophane.
Organic matter held in soil pores
1
2.3.5.2
m diameter in clay soils (sometimes called
“sterile pores”) is also less accessible to microbial attack. Cultivation tends to break
down soil structure so that organic matter in small pores is exposed to microor-