Agriculture Reference
In-Depth Information
3
O
6
-
Al Si
O
4
(OH)
2
+
6
(MgFeAI)
O
4
(OH)
2
1. 4 3
nm
++
Mg
3
O
6
-
Al Si
H
2
O
++
++
Mg
Mg
H
2
O
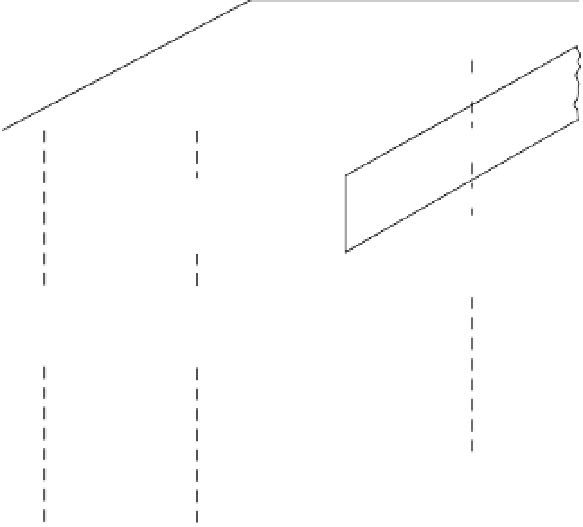
Structure of vermiculite crystals. The basal spacing is fixed in the presence of partially
hydrated Mg
2+
ions in the interlayer spaces (White 1997). Reproduced with permission of
Blackwell Science Ltd.
Figure 2.7
Vermiculites
have both di- and trivalent cations occupying all the available
sites in the alumina sheet, producing a net positive charge that partly neutralizes
the negative charge developed through substitution of Al
3
for Si
4
in the silica
sheet. The polarizing effect of the tetrahedral charge is lessened, so that the cations
Ca
2
and Mg
2
in the interlayers are only partially dehydrated. The basal spac-
ing of a Mg-vermiculite, for example, is typically 1.43 nm, as shown in figure 2.7.
This collapses to 1 nm on heating to drive off the water, so the vermiculites show
limited reversible swelling. In an acid environment, hydrated Al
3
ions replace
Ca
2
and Mg
2
in the interlayers and “islands” of Al(OH)
3
form as the Al
3
ions hydrolyze and polymerize. This gives rise to an aluminous
chlorite
type of
clay mineral.
Smectite
minerals have a varied composition, with isomorphous substitution
occurring in both the silica and alumina sheets. The most common smectite in
the soil,
montmorillonite
, has substitution in the alumina sheet only, usually Fe
2
or Mg
2
for Al
3
(table 2.2). The interlayer cations are also partially or fully hy-
drated (see box 2.6) and freely exchangeable with other cations in solution. De-
pending on the type of exchangeable cations, the basal spacing may vary from 1.5
to 4 nm, as illustrated in figure 2.8. Ca-montmorillonite, a form common in the
soil, has a basal spacing of 1.9 nm at full hydration, when there are three layers
of water molecules between the crystal layers. The stacking of the layers is very ir-
regular and the average crystal size is much smaller than in the micas and kaolin-
ites. The interlayer surfaces provide a large internal area that adds to the external
planar area, so the specific surface area of smectite clay is very large (table 2.1).
Because of the weak interlayer bonding and the free movement of water and
cations in and out of this region, the smectites are called
expanding-lattice clays
.