Agriculture Reference
In-Depth Information
The molecular structure of feldspars is introduced in box 2.3. Their structure
consists of polymerized Si tetrahedra in which some Si
4
is replaced by Al
3
. The
cations balancing the excess negative charge are commonly K
, Na
, and Ca
2
and less commonly, Ba
2
and Sr
2
. They are chemically more reactive and more
easily weathered than silica minerals, and so rarely comprise more than 10% of
the sand fraction of mature soils.
Minerals of the Clay Fraction
These minerals are subdivided into the
crystalline clay minerals,
predominantly
phyllosilicates, and
accessory minerals
, which are salts, oxides, carbonates, and re-
sistant primary minerals reduced to a very small size by weathering. Because of
their large specific surface areas and surface charges (table 2.1), these minerals pro-
vide very important sites for reactions with nutrients and water in soil (see chap-
ters 4, 5, and 6). The clay minerals are conveniently classified on the basis of their
Si:Al mole ratios.
2.2.4
1
The most common mineral of this group is
kaolinite
, which is found in many
highly weathered soils. Kaolinite has a 1:1 layer-lattice structure formed by the
sharing of O atoms between a silica sheet and an alumina sheet. The basal spac-
ing of the crystals is fixed at 0.72 nm as a result of “hydrogen bonding” between
the H and O atoms of adjacent layers (figure 2.6). The layers stack fairly regu-
larly in the
c
direction to form large crystals 0.05-2
2.2.4.1
Minerals with a Si:Al Mole Ratio
m thick. Because of the hy-
drogen bonding between layers, water and solute molecules do not penetrate the
interlayer spaces, and the specific surface area is low (table 2.1). Kaolinite clays
show minimal shrinkage or swelling with a change in water content. The mineral
halloysite
has the same structure as kaolinite, except for the presence of two sheets
of water molecules between crystal layers, which causes the layers to curve and
form a tubular crystal. Halloysite is found in weathered volcanic ash soils.
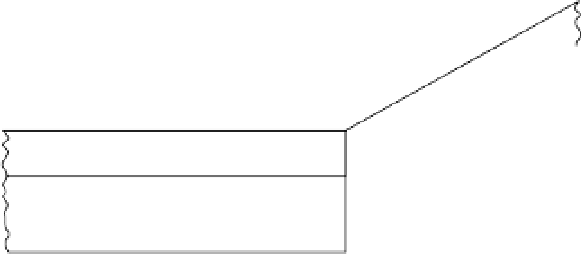
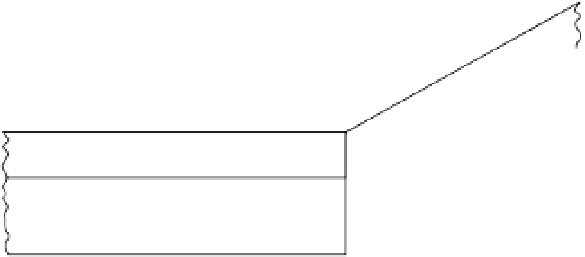
Structure of a kaolinite crystal with hydrogen bonding between layers (White 1997).
Reproduced with permission of Blackwell Science Ltd.
Figure 2.6
0.72
nm
O
2
(OH) Al
2
(OH)
3