Agriculture Reference
In-Depth Information
(a)
(b)
Oxygen
Aluminum
Silicon
Hydroxyl
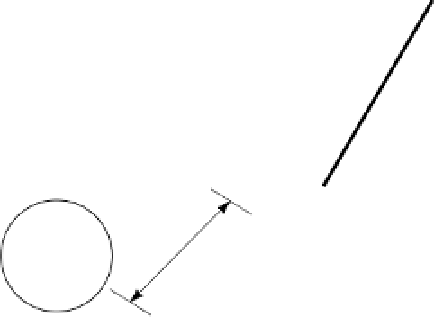
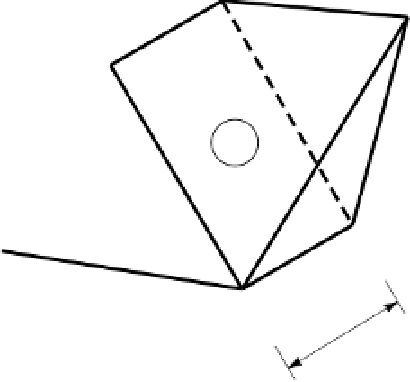
Figure 2.4
(a) Diagram of a Si tetrahedron. (b) Diagram of an Al octahedron (interatomic distances
not to scale) (White 1997). Reproduced with permission of Blackwell Science Ltd.
2.2.3.2
Chains and Sheets
Silicate minerals are formed by linking together the basic silicon and aluminum
units (a process of polymerization). This occurs by covalent bonding of O atoms
to form chain, sheet, and three-dimensional structures. In the
pyroxene
minerals,
for example, each Si tetrahedron is linked to adjacent tetrahedra by sharing two
Box 2.3
Electric Charges and Valency
Oxygen in its ionic form O
2
has two “free” electrons. Normally, the free
electrons are shared between two O atoms to form an uncharged molecule, O
2
.
When four O
2
ions are coordinated to one Si
4
ion to form a tetrahedron (fig.
2.4a), the deficit of electrons associated with Si is satisfied by four electrons from
the oxygens. But this leaves four negative charges potentially unneutralized. Such a
charge imbalance cannot exist in nature, so cations such as Al
3
, Fe
3
, Fe
2
,
Ca
2
, Mg
2
, K
, and Na
are attracted to the SiO
4
4
units. The cations become
covalently bonded to the O atoms so that the surplus charges on the O atoms in
the SiO
4
4
unit are neutralized. An electrically neutral silicate crystal called a
feldspar
is formed.
The number of unneutralized charges (
or
) associated with the ionic
form of an element defines its valency state. Elements of similar size and the same
valency frequently substitute for one another in a silicate structure—a process
called
isomorphous substitution
. The structure remains electrically neutral. However,
when elements of similar size but different valency exchange, there is an imbalance
of charge. The most common substitutions are Mg
2
or Fe
2
for Al
3
in
octahedral sheets, and Al
3
for Si
4
in tetrahedral sheets (see section 2.2.3.2). The
excess negative charge is neutralized by the incorporation of additional cations into
the crystal lattice or by structural arrangements that allow an internal
compensation of charge (e.g., in the chlorites).