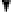Agriculture Reference
In-Depth Information
Adsorption Forces
. In very dry soils (relative humidity, RH, of the soil air
20%), water is adsorbed onto the clay and silt particles as a monolayer in which
the molecules are hydrogen bonded to each other and the surface. With an in-
crease in RH, more water molecules are adsorbed by hydrogen bonding to those
on the surface. The charged surfaces of clay minerals also attract cations, and the
electric field of the cation orients the polar water molecules around the ion to
form a hydration shell, containing 6-12 water molecules. Work is done in at-
tracting water molecules to a cation, which means there is a reduction in the free
energy of water in each hydration shell.
Capillary Forces
. As water layers build up in a dry soil, water is drawn by sur-
face tension forces into the narrowest pores and wedges between soil particles,
forming curved air-water menisci (fig. 6.1a). The effect on the soil water is ex-
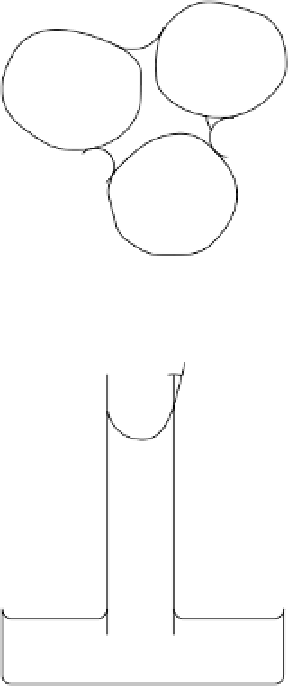
actly analogous to the rise of water in a glass capillary tube inserted into a dish of
water (fig. 6.1b). The decrease in free energy as the surface tension force draws
water up the tube is balanced by the increased potential energy of the water col-
umn. Atmospheric pressure acts on the upper surface of the water in the capillary
tube and also on the free water surface outside the tube. Thus, the pressure dif-
ference
P
across the meniscus is equal to the decrease in free energy of the wa-
Soil
P
(a) Water held by surface tension
forces in narrow pores between soil
particles (b) Water drawn up a glass
capillary tube by surface tension
forces (White 1997). Reproduced
with permission of Blackwell Science
Ltd.
Figure 6.1
Air-water
particle
meniscus
α
Radius,
r
(a)
P
r
α
Difference in
pressure,
∆
P
Height, h
Atmospheric
pressure,
P
A
(b)