Agriculture Reference
In-Depth Information
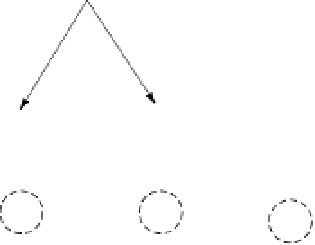
Nonexchangeable K
Wedge-
shaped
site
K
+
K
+
K
+
Figure 4.8
Exfoliation of a micaeous clay on
weathering to form “wedge-shaped”
sites that hold K+ ions (White 1997).
Reproduced with permission of
Blackwell Science Ltd.
K
+
K
+
K
+
The micronutrient cations Cu
2
, Fe
3
, Mn
2
, Zn
2
, and Co
2
are also
classed as “nonexchangeable” when they form chelates with organic compounds
(section 2.3.4.2). Similarly, when the nutrient anions of P, Mo, and B are specif-
ically adsorbed on clays or oxides, they are not readily exchanged by anions such
as Cl
and NO
3
. But as discussed in section 4.5.4, they are exchanged by other
specifically adsorbed anions supplied at a high concentration in solution, or when
the pH is raised. These forms of cations and anions comprise a nutrient pool of
intermediate availability to plants—intermediate between the readily exchange-
able forms and the insoluble precipitates, or unweathered rock minerals.
Exchangeable Al
3
and Soil Acidity
4.6.3
4.6.3.1
Acid Clays
A soil becomes acidic as Ca
2
, Mg
2
, K
, and Na
ions are leached faster than
they can be replaced by mineral weathering and atmospheric inputs. Initially, the
exchangeable cations are replaced by H
ions, which are continually generated
from carbonic acid (H
2
CO
3
) formed by CO
2
dissolving in the soil water:
CO
2
H
2
O
H
2
CO
3
H
HCO
3
H
CO
3
2
(4.14)
Pure rainwater in equilibrium with CO
2
in the atmosphere has a pH of 5.65.
But as respiration increases the partial pressure of CO
2
in the soil air, the reac-
tions are driven to the right, producing more H
ions. The intensity of acidity
that develops is measured by the soil pH, as outlined in box 4.6.
In soil, the small mobile H
ions invade clay mineral lattices. At a pH
4,
mineral weathering is accelerated, releasing Al, SiO
2
, and smaller amounts of Mg,
K, Fe, and Mn. The SiO
2
combines with water to form weak silicic acid (Si(OH)
4
),
which leaches away, and the Al, Mg, K, and some Mn are retained, initially as ex-
changeable cations. With the exception of soils containing illitic clay, K
is lost
by leaching, and the clay that remains is dominated by Al
3
with some Mg
2
(fig. 4.9). Hence an
acid clay
becomes an Al-clay. The hydrated Al
3
ions are ex-
changeable, and the amount present is usually measured by displacement in M
KCl solution. Some H
ions, produced by the hydrolysis of the Al
3
ions (see
next section), are also displaced. The sum of exchangeable Al
3
and H
defines
the soil's
exchangeable acidity
(in cmol H
/kg).