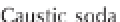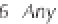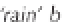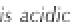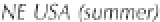Geology Reference
In-Depth Information
Box 3.2
pH AND Eh
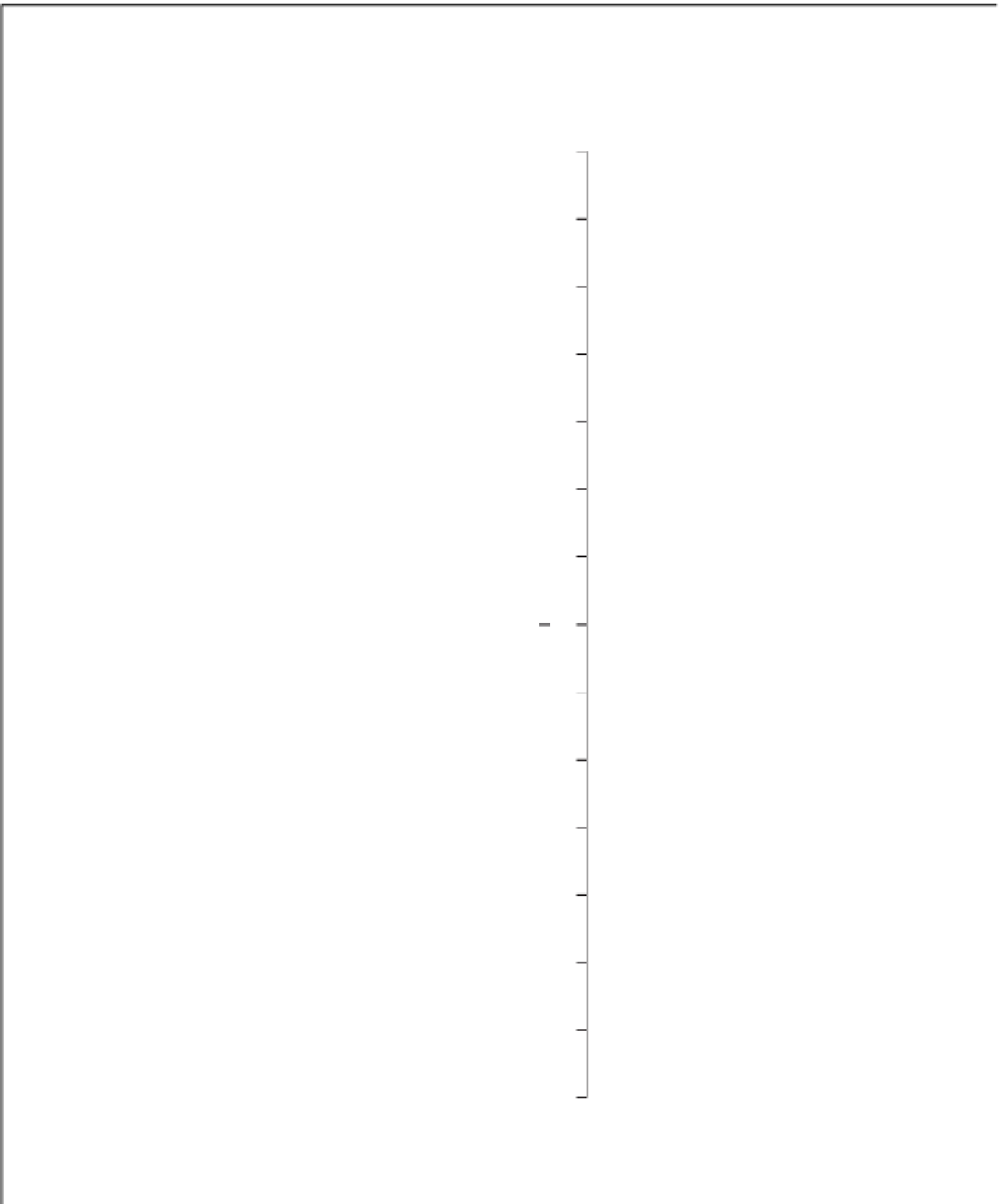
pH
is a measure of the
acidity
or
alkalinity
of aque-
ous solutions. The term stands for the concentration
of hydrogen ions in a solution, with the p stand-
ing for
Potenz
(the German word for 'power'). It is
expressed as a logarithmic scale of numbers rang-
ing from about 0 to 14 (Figure 3.2). Formulaically,
pH
, where [H
+
] is the hydrogen ion
concentration (in gram-equivalents per litre) in an
aqueous solution. A pH of 14 corresponds to a hydro-
gen ion concentration of 10
−
14
gram-equivalents per
litre. A pH of 7, which is neutral (neither acid nor alka-
line), corresponds to a hydrogen ion concentration of
10
−
7
gram-equivalents per litre. A pH of 0 corresponds
to a hydrogen ion concentration of 10
−
0
H
+
]
=−
[
log
1) gram-
equivalents per litre. A solution with a pH greater than
7 is said to be alkaline, whereas a solution with a pH
less than 7 is said to be acidic (Figure 3.2). In weather-
ing, any precipitation with a pH below 5.6 is deemed
to be acidic and referred to as '
acid rain
'.
The solubility of minerals also depends upon the
Eh
or
redox
(
reduction-oxidation
)
potential
of a solu-
tion. The redox potential measures the oxidizing or
reducing characteristics of a solution. More specifically,
it measures the ability of a solution to supply electrons
to an oxidizing agent, or to take up electrons from a
reducing agent. So redox potentials are electrical poten-
tials or voltages. Solutions may have positive or negative
redox potentials, with values ranging from about
(
=
−
0.6
+
volts to
1.4 volts. High Eh values correspond to oxi-
dizing conditions, while low Eh values correspond to
reducing conditions.
Combined, pH and Eh determine the solubility
of clay minerals and other weathering products. For
example, goethite, a hydrous iron oxide, forms where
Eh is relatively high and pH is medium. Under high
oxidizing conditions (Eh
100 millivolts) and a
moderate pH, it slowly changes to hematite.
>
+
Figure 3.2
The pH scale, with the pH of assorted
substances shown.