Biology Reference
In-Depth Information
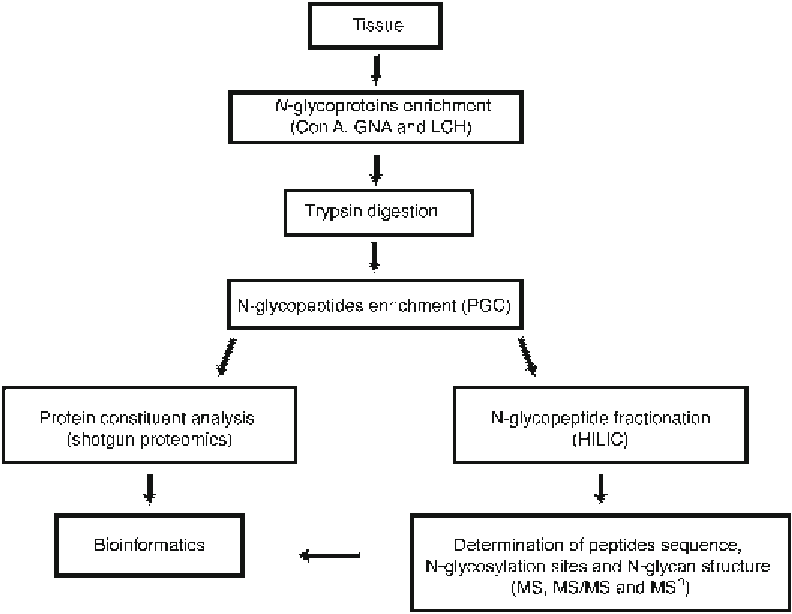
Fig.
1
Strategy workfl ow for systematic study of
N
-glycoproteins
site occupancy, and interpretation of glycan structure and glycoforms
(Fig.
1
). However, several factors can complicate the glycoprotein
analysis, including the complexity of biological samples and low
protein abundance. Therefore, the reduction of the complexity
and enrichment of glycoproteins can represent an effective fi rst
step. This can be accomplished by lectin affi nity chromatography
[
15
-
17
] and chemical methods, such as hydrazine chemistry [
18
]
and boronic acid [
19
]. However, lectin affi nity chromatography
with Concanavalin A has been more commonly used for studies of
plant glycoproteins [
15
-
17
] and a number of glycoproteomic analy-
ses have been reported in the last few years employing lectin affi nity
as an early enrichment step [
20
], including those that use multiple
lectins to increase the population of captured glycoproteins [
17
]. To
this end, a range of lectins with different affi nities is now commer-
cially available (Table
1
). However, the chemical methods should be
considered as complementary analytical approaches for a systematic
study [
14
,
21
], although they are not further discussed here.
In this chapter we present a protocol to enrich for
N
-glycoproteins
from plant tissues using lectin affi nity chromatography, and to pre-
pare the samples for downstream experiments designed to identify
the proteins using mass spectrometry (MS).