Biology Reference
In-Depth Information
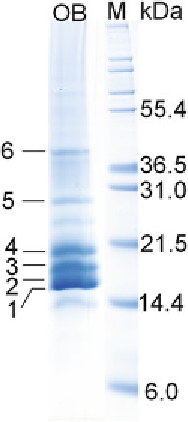
Fig. 1
SDS-PAGE protein profi le of isolated OBs. Bands
1
-
6
, corresponding to OB
proteins, were excised and analyzed with LC-MS/MS. The OB lane is intentionally
overloaded, to increase the protein concentration. This is also the reason why the
other, nonspecifi cally bound proteins are visible at the OB profi le (but in lower
concentrations).
M
—protein standards (marker)
8. For further LC-MS/MS analysis, distribute 100
L of mixed
OB suspension into Eppendorf tube. For each proteolytic
system, one tube with 100
μ
L of OBs is needed. Prepare three
samples of OB suspension for all three proteolytic systems.
9. Spin the samples at 20,000 ×
g
for 20 min at 4 °C. Using an
injection needle coupled with a syringe, remove the suspension
buffer bellow the OB layer. Leave the OBs in the tube and add
100
μ
L of PLB. Resuspend the OBs in the PLB by tapping the
Eppendorf tube over the edges of an Eppendorf tube stand.
This simple pre-concentration step allows loading higher
protein amounts on SDS-PAGE.
10. Heat the samples at 72 °C for 10 min (
see
Note 14
).
11. Load the samples on the gel. 100
μ
L of the sample should be
loaded into four lanes (
see
Note 15
).
12. Add protein standards in one lane at each gel.
13. Run the electrophoresis under 160 V till the bromophenol
blue dye front has reached the bottom of the gel (usually 1 h).
14. Turn off the electrophoresis and open the gel plates with a
spatula. Rinse the gel with water and transfer carefully to a
container.
μ