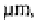Agriculture Reference
In-Depth Information
The
heavy fraction
of soil organic matter is composed of humic substances which are
linked to mineral constituents to form organo-mineral structures with bulk densities of
more than 2.0 Mg Humic substances are characterised by having a high proportion
(
ca.
50 % or more) of their carbon atoms incorporated within aromatic structures.
At least part of the humic acid content may originate from such highly degradation-
resistant phenolic materials as lignins and vacuolar polyphenols contained in the original
plant material. Clay minerals may be the catalysts for such chemical condensation,
perhaps by promoting the polymerisation of phenolic ring structures resistant to
biological attack (Wang
et al
., 1980).
Humic substances are characterised by their solubilities in certain extractants and
the following three fractions are defined:
(i) humin, which is insoluble in
(ii) humic acids which are soluble in these extractants but are precipitated when
the solution is acidified;
(iii) fulvic acids which remain in solution following acidification.
Fulvic acids have low molecular weights (1,000-30,000 Da) and variable shapes,
depending on their bonds with other inorganic and organic soil constituents (Paul and
Clark, 1989). Humic acids have higher molecular weights (10,000-100,000 Da) and are
formed by the poly-condensation of elements containing a considerable proportion of
their masses as aromatic rings, nitrogen in cyclic form and peptide chains. Humin is
a mixture of humic and fulvic acids with some plant and microbial remains, all linked
by clay minerals.
Variation in the chemical composition of humic acids has been related to annual
rainfall, the nature of the land use and is known to differ between soils (Kumada, 1987;
Almendros
et al
., 1988; Arshad and Schnitzer, 1989). However, climatic differences
(notably temperature), may not always be reflected in the composition of humic acids
(Shoji
et al
., 1987).
Recent characterisation of soil organic matter using solid-state spectroscopy and
other techniques is yielding useful information on the distributions of different groupings of
chemical compounds in space, in depth and with changes in land use. Gregorich
et al.
(1996)
compared the chemical compositions of surface-soil organic matter under a mixed-species
hardwood forest and 90 years after conversion to maize (
Zea mays
) cropping. In the maize
system, the surface soils were relatively depleted in C, N and in a number of high
molecular weight compounds, particularly lipids and lignin. The compositions of the light
fractions of both systems reflected those of the vegetation from which they were derived,
apart from smaller amounts of carbohydrates and greater quantities of sterols, indicating that
some decomposition had taken place. Compared with more decomposed materials greater
than 53 the light fraction had greater carbohydrate and aliphatic material but less
aromatic material. Higher concentrations of lipids and lignin monomers and dimers were also
present in the light fraction. No differences were apparent between the subsoils and the
subsoil organic matter was almost free of carbohydrates, phenols and most lignins.
The compounds present at depth were the most recalcitrant and included alkyl
aromatics, complex nitrogen compounds, lignin residues and small quantities of lipids.
Chemical changes following cultivation are largely associated with the light fraction
(Skjemstad
et al
., 1997).