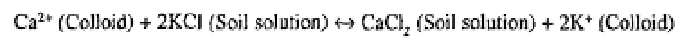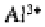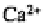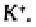Agriculture Reference
In-Depth Information
this is succeeded by a more diffuse layer of cations further away. Cation concentrations
are thus high close to the surfaces and decrease with increasing distance from the
surface to a level similar to that of the soil solution. Anion concentrations may vary
in an inverse manner.
Net exchanges occur when changes in ionic concentrations or pH occur in the soil
solution. These may result from seasonal changes in soil moisture or through the addition
of fertilisers or other soluble amendments. For example, the addition of KCl as a fertiliser
may cause a displacement of calcium ions into the soil solution (Duchaufour, 1997):
As indicated in the above equation, such reactions are reversible; they also take place in
terms of chemical equivalents. That is, one centimole of potassium ions will exchange
for one centimole of calcium ions in the above exchange reaction.
Not all ions are adsorbed equally onto the colloid surfaces. With a few exceptions,
the ions of higher valency are held more tightly than those of lower valency. Thus, in
decreasing order, is held more tightly than which is held more tightly than
For ions of equal valency, the least hydrated ions are preferentially adsorbed. ions
are preferentially adsorbed onto organic colloids and this ion therefore dominates in soils
where organic matter concentrations are high.
Where exchangeable Na ions comprise 6-15 % or more of the cation exchange
capacity (sodic soils), the large radius of the hydrated Na ion may move adjacent
phyllosilicate colloid surfaces apart to the extent that deflocculation occurs. This leads
to considerable instability in the soil and sodic soils are subject to severe erosion, surface
sealing and the development of strong surface crusts together with other phenomena
inimical to ecosystem function and productivity. The importance, morphology, structure
and distribution of crusted soils are discussed in the volume edited by Sumner and
Stewart (1992).
Not all reactions between ions in the soil solution and clay surfaces can be explained
in terms of simple exchange phenomena. A range of cations, phosphate and other anions
may all be subject to an initial adsorption process followed by slow diffusion into,
particularly, iron oxides such as goethite (Barrow, 1989).
Table I.6 presents examples of the cation exchange capacities of a range of soils
classified at the ordinal level of Soil Taxonomy (Soil Survey Staff, 1999). A number of
points are illustrated by the table. Firstly, considerable differences exist between the
orders, with the vertisols having the highest overall values due to the expansive (smectitic)
clay minerals present in these soils. With increasing depth in the soil, a clear reduction
in cation exchange capacity occurs paralleling the substantial decline of organic matter
concentrations with depth. Finally, considerable differences exist between estimates of
cation exchange capacity depending on the measurement methodology. This is of
particular importance in soils where variable charge components dominate, notably
in such acid, highly-weathered soils as the ultisols, oxisols and exemplified here by
the rainforested inceptisol (Cannon
et al
., 1992) and the spodosol described by Mew
and Lee (1981). Because of the variable charge components present, inflated estimates
of cation exchange capacity result, particularly in acid soils where substantial differences