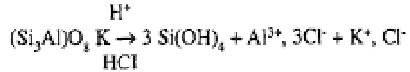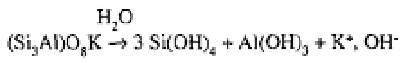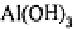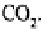Agriculture Reference
In-Depth Information
Neutral hydrolysis
Hydrolysis is the degradation of aluminosilicate minerals by the free or ions of
water in weak saline solutions and at pH values between 5 and 9.6; it is accelerated by
the presence of dissolved As an example, the hydrolysis of feldspars may be writ-
ten as follows (Pédro and Sieffermann, 1979):
where the
occurs in the form of the crystalline solid gibbsite.
Acidolysis
Acidolysis occurs in the presence of such acid solutes as the organic acids released from
biological materials; the reaction is:
where all components are soluble.
Through these reactions, the primary silicates are broken down, dissolved and their
constituents transformed into secondary minerals (silicate clays, oxides, hydroxides).
Pédro (1964) showed in laboratory weathering experiments simulating tropical condi-
tions, that a variety of parent rock materials (basalt, granite and andesitic lava) are rapidly
hydrolysed to form secondary sesquioxides (
i.e.,
Fe and Al oxides and hydroxides)
within a few months. In contrast, crushed granite placed in lysimeters under simulated
temperate climate conditions showed a significant microdivision of the rock, but no
mineralogical modification. Hydrolysis therefore appears to be the dominant process
occurring under conditions of high environmental temperatures and available free water.
Partial acidolysis may lead to aluminization, a transformation in which the 2:1 clay
sheet structure is preserved leading to aluminized clays (Pédro, 1989).
Acido-complexolysis
Acido-complexolysis occurs when organic acid components (simple aliphatic or fulvic
acids) form mobile soluble complexes with the Fe and Al of the primary clay minerals
and impede the formation of secondary minerals. This is the characteristic mode of
weathering in the spodosols.
Salinolysis
In highly saline solutions, hydrolysis is replaced by salinolysis in the pH range of 5
to 9.6 (chlorides or sulphates) and alkalinolysis (solonisation) where the pH is greater
than 9.6 (carbonates).