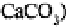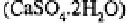Agriculture Reference
In-Depth Information
Calcium dominates the exchange complex of most well-structured soils where it exists
in an equilibrium state with the soil solution. Site losses occur through erosion, illuviation,
leaching, subsurface lateral flows, and plant uptake. It is the dominant metallic element
in areas dominated by silicate rock parent materials (Schlesinger, 1997).
Soil pH is strongly influenced by calcium concentration and it therefore indirectly
affects the supply of a number of nutrient elements. At high pH, for example, the avail-
ability of many of nutrient elements is severely reduced. In particular, phosphorus
availability is substantially reduced at pH values above 7, largely by fixation on inorganic
calcium minerals. Calcium promotes the flocculation of soil colloids and in agricultural
and other environments where it dominates the exchange complex, a well-developed
aggregate structure favourable to aeration and water infiltration normally occurs.
In contrast, magnesium (together with sodium) may have a dispersive effect.
Applied aspects
Because of the acid-producing effects of nutrient uptake, the removal of plant parts in
crops and the losses of calcium through erosion, leaching and the use of acidic fertilisers,
intensively-used agricultural lands tend to become progressively acidified. The applica-
tion of calcium as agricultural lime to raise soil pH is therefore a widespread and
regularly-required feature of intensive agricultural practice. The primary purpose of this
is to raise the soil pH to the range in which most nutrient elements are readily available
(Figure I.35) although the calcium supplied is also available as a nutrient element.
Calcium may be applied to the soil as agricultural lime in carbonate, oxide or
hydroxide form. Crushed limestone (largely is the most frequently used medium;
limestones may also contain a variable proportion of dolomite depending
on their sources. Calcium is also applied to soils as gypsum to alleviate
the high dispersivities and often crusted states characteristic of sodic soils. The dispersive
nature of these soils is reduced by replacing a proportion of the sodium ions with
leading to improved flocculation of the soil colloids, lessened dispersivities and a reduced
tendency to form surface crusts.
3.1.2.6
Potassium
Potassium is required by all living organisms, normally in relatively large amounts.
Its major roles in plants are in pH stabilisation, osmoregulation, enzyme activation and
membrane transport processes; these impact on protein synthesis, photosynthesis and
many other basic processes at both the cellular and physiological levels (Marschner,
1995). Potassium is very mobile in plants at the level of the cells, the tissues and at
the longer distance transport level of the phloem and the xylem; it is thus readily
redistributed in response to changing metabolic requirements. Leaves usually have higher
concentrations than the other plant parts (Table I.15) and potassium is, after
calcium, the most abundant metallic element in the cytoplasm of the leaves. Fertilised
crop plants in particular may take up substantially .more than their metabolic
requirements, that is, so-called 'luxury' consumption. Potassium is not an important
component of the structural tissues.