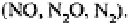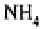Agriculture Reference
In-Depth Information
land and lateral subsurface flows. In soils subject to excessive nitrogenous fertiliser
application‚ nitrates may leach through the soil to depths below the range of the roots
and contaminate local aquifers. Nitrate is toxic in excess and health problems may ensue
when contaminated waters are consumed by humans or domestic stock (Spalding and
Exner‚ 1993) (see also IV.2.6.2).
Substantial quantities of nitrogen may be lost from ecosystems in other forms and
losses of through volatilisation and from living plants‚ and of nitric oxide (NO) and
nitrous oxide during nitrification may all be significant. Denitrification occurs in
anaerobic soils and in anaerobic microsites within aggregates‚ even within otherwise
well-aerated soils (Figure I.15) leading to the loss of denitrification gases
Such 'greenhouse' gases are of importance in global climate change scenarios.
Ammonia volatilisation occurs through the hydrolysis of ammonium compounds and
may be a source of major nitrogen losses where such compounds are applied as fertilisers
to agricultural systems‚ particularly in alkaline soils. More than 50
per cent.
of the nitro-
gen applied may commonly be lost‚ depending on the chemical form of the fertiliser and
the depth of its application‚ the temperature and the buffer capacity of the soil (Peoples
et al
.‚ 1995). Nitrogen losses from animal secreta and from alkaline areas where nitroge-
nous fertilisers have been applied can be particularly severe in certain grazing systems
(Jenkinson‚ 1990). In environments where biomass burning is frequent‚ nitrogen losses
through volatilisation are also likely to be substantial (Vitousek and Howarth‚ 1991).
Since
ca.
90 % of soil nitrogen reserves are organic (Sowden
et al
.‚ 1977)‚ the distri-
butions of nitrogen and carbon are usually closely correlated. This is apparent in the
similarities of the frequency distributions of Figures 1.32a and 1.32b which compare
the frequency distributions between carbon and nitrogen in the A horizons of 2099
Australian surface soils. Figures 1.33a-f present the related depth distributions of carbon
and nitrogen in the six selected soils discussed in the previous section. In most of these‚
the general nature of the concentration decline with depth is similar although the C:N
ratio clearly alters with depth in the alfisol and the spodosol. In contrast‚ the histosol
has a characteristically higher C:N ratio throughout although it varies somewhat within
both the peaty O and the mineral-dominated horizons.
Soil organic nitrogen comprises
ca.
40 % proteinaceous materials (proteins‚ peptides‚
amino acids)‚ 5-6 % amino sugars‚
ca.
35 % heterocyclic compounds (including purines
and pyrimidines) and
ca.
19 % -N (Schulten and Schnitzer‚ 1998). Some geographic
variation has been detected with‚ for example‚ amino acid and amino sugar N forming
a greater proportion of total N in soils from cooler areas (Sowden
et al
.‚ 1977). Much soil
organic N appears to be protected in associations with humic materials‚ clay or the hydrous
oxide minerals.
3.1.2.3
Phosphorus
Phosphorus is a further major nutrient element required by all forms of life. It plays an
important role in energy metabolism (through its involvement in ATP and ADP) and in
a range of metabolic pathways. It is a structural component of coenzymes‚ phospho-
proteins and phospholipids; as a component of nucleic acids‚ it forms a part of the genetic
information system. In higher animals it plays an important additional structural role in