Agriculture Reference
In-Depth Information
et al
.‚ 1993; Jones and Darrah‚ 1994). Micro-organisms may also take up nitrogen in
organic form. For example‚ all fungi appear able to utilise some organic nitrogen sources
while certain plant pathogenic fungi may be unable to utilise inorganic forms (nitrate or
ammonium) (Jennings‚ 1989). Ericoid‚ some ectomycorrhizal and other fungi (notably
wood-rotting species) produce diffusible enzymes around the roots and assimilate the
lower molecular weight breakdown products of protein degradation (Read
et al
.‚ 1989)
to meet their nitrogen (and sulphur) requirements (Chapter IV.3.1.2.2).
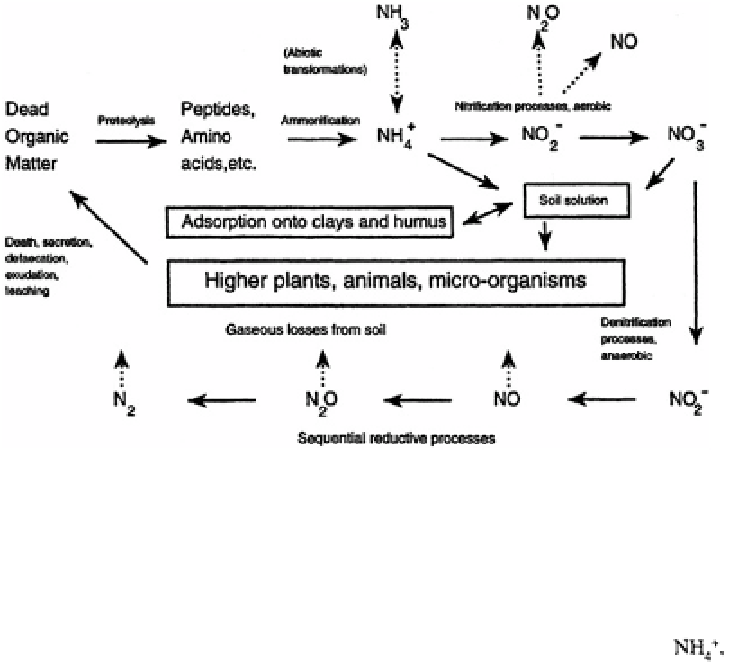
Nitrogen transformations (and other plant assimilation processes) have clearly different
effects on soil pH. Assimilation of nitrogen as (and other positively charged ions)
by both plants and micro-organisms leads to proton production and soil acidification‚
while assimilation as results in hydroxyl or bicarbonate ion production. European
examples of the former group (acidophiles) are pines (
Pinus
spp.) and spruces (
Picea
spp.); these species are well adapted to acid soils and take up their nitrogen as
In contrast‚ neutrophiles are species which are adapted to less acid soils and take up
nitrogen as nitrate; they include the elms (
Ulmus
spp.)‚ birches (
Fraxinus
spp.) and the
maples (
Acer
spp.). The oaks (
Quercus
spp.) and the beeches (
Fagus
spp.) appear less
exigent in terms of soil pH and may take up both forms of nitrogen (Duchaufour‚ 1997).
Overall‚ nitrification and denitrification processes also have‚ respectively‚ acidifying and
neutralising effects (Sprent‚ 1987).
The mobilities of nitrate and ammonium ions in soils differ substantially. While
ammonium ions are held at the negatively charged surfaces of clays and organic colloids‚
nitrate does not interact appreciably with the exchange complex and moves readily in
the soil solution. It is thus susceptible to loss off site through deep leaching or in over-