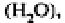Agriculture Reference
In-Depth Information
species) may also accumulate substantial quantities of certain elements‚ some of which
may cause problems in animal nutrition through food chain effects. For example‚ in
certain accumulator plants growing in seleniferous soils‚ selenium may occur at
concentrations that make them toxic to grazing animals (Marschner‚ 1995).
Certain plants (extreme metallophytes) may accumulate extraordinarily large amounts
of metallic elements. Baker and Walker (1990) report the following concentrations of
metals in the foliage of various species of vascular plants: Cu 1.2 %‚ Co 1.0 %‚ Mn 5.1 %‚
Ni 4.7 %‚ Pb 0.8 % and Zn 3.9 % of dry biomass. Accumulation of metals by certain
plants and sometimes animals (indicator species) has been used by economic geologists
to indicate the presence of subterranean orebodies (Brooks‚ 1983); such accumulations
may also be indicative of contaminated sites.
Of the elements needed by higher organisms‚ some‚ the
macro-nutrients
‚ are
required in relatively large quantities. Macro-nutrients usually have relatively
well-defined roles in plant biology; for example‚ calcium is a major structural compo-
nent of the cell walls of higher plants where it occurs in conjunction with pectin‚ while
magnesium is a central element in the structure of chlorophyll‚ a magnesium porphyrin.
In contrast‚ the
micro-nutrients
are needed in only very small amounts although‚
as indicated above‚ they too may accumulate in plant tissues in concentrations much
greater than dictated by physiological requirements. Not all higher plants require all
the recognised nutrient elements. Sodium‚ for example‚ is a recognised micronutrient
only for those plants that possess C4 or crassulacean acid metabolism photosynthetic
pathways‚ and some cyanobacteria (Brownell‚ 1979).
The nutrient elements may also be classified into four major groups based on the
principal roles that they fulfil in plant tissues (Table I.16) (Mengel and Kirkby‚ 1987).
The group I elements‚ including carbon‚ hydrogen and oxygen are the principal
structural components of all living tissues and play important roles in enzymes and other
functional groups. They are taken up as liquids
gases
or as
ions from the soil solution (for example‚
Members of group II are taken up from the soil solution as the oxyanions - phosphate
borate or boric acid and silicate. The phosphate anion is particularly critical to energy
transfer mechanisms within the plant while boron is important in membrane function.
The group III elements are taken up from the soil solution and play osmotic and ion
balance roles within plant tissues. Other roles include more specific functions in enzyme
conformation and catalysis. Clarkson and Hanson (1980) state that members of this group
occur largely as free or reversibly-bound ions rather than as metallo-proteins. Members
of group IV occur in plants as structural chelates or metallo-proteins; Fe‚ Cu and Mo (and
Mn) participate in redox reactions. Uptake occurs in the form of ions or as chelates from
the soil solution. Table I.16 presents the forms assumed in plant tissues by a range of nutri-
ent elements and‚ for those important in forming salts and complexes‚ some major roles.
While many nutrient elements may accumulate in plant tissues at concentrations
above physiological requirements‚ most become toxic when present in considerable
excess. Although complicated by interactions with other elements and growth-related
factors‚ individual concentrations may be considered to lie within a spectrum of levels
which run from deficient to suboptimal to optimal to supraoptimal to toxic (Smith‚
1962)‚ a concept also pertinent to animal nutrition.