Environmental Engineering Reference
In-Depth Information
10000
1000
0.2
Available Content
Leachate
Solubility
QA/QC check
for leachate
saturation
availability limited
higher diffusivity
0.15
100
10
1
0.1
lower diffusivity
change in
release control
0.05
0.1
0.01
0
0.1 1 10 100
1 2 3 4 5 6 7 8
Time [days]
Leachate #
a)
b)
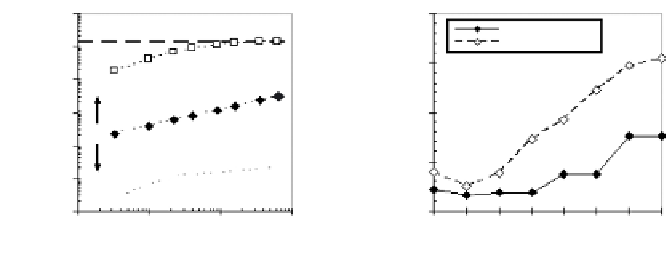
FIGURE 10.8
Mass-transport rate test data consistency: a) the effect of release mechanisms
on cumulative release data and b) comparison of leachate concentrations to solubility con-
straints as a function of leachate pH.
(iii) constituent depletion does not occur, and (iv) matrix degradation does not
significantly alter the matrix properties influencing leaching.
The determination of a characteristic diffusivity using release information is
somewhat uncertain because of ambiguity in the representation of the diffusion
coefficient. The relationship between observed, effective, and molecular diffusivity
218
is shown in the following expression:
eff
mol
D
R
D
obs
(10.3)
D
=
=
⋅
τ
R
Molecular diffusivity,
D
mol
, is the term denoting the rate of change in the con-
centration gradient for a species diffusing in an aqueous media. Diffusion in a non-
reactive porous medium retards the release of constituents in relationship to molec-
ular diffusion through effective surface area and increased path length, or tortuosity τ.
The effective diffusivity,
D
eff
,
is used to describe the mass transport when physical
retardation is present. In chemically reactive systems, the rate of diffusion for a
constituent is slowed due to interactions with the surface, or chemical retardation R.
One measure of diffusivity in chemically reactive systems is the observed, or appar-
ent, diffusivity
D
obs
.
10.5.2.2
Regression of Diffusivity from Leaching Data
The most common method for calculating diffusivity from leaching data assumes
that release of constituents is diffusion-controlled with constant observed diffusivity
and that a simple diffusion model may be used to describe constituent release. The
diffusion model is characterized by two parameters; the initial concentration of the
constituent in the waste form and the diffusivity of the constituent through the solid
matrix. The driving force for diffusion is the difference between the constituent
concentration in the bulk solid phase and the leachate.













Search WWH ::

Custom Search