Environmental Engineering Reference
In-Depth Information
100000
10000
1000
100
10
1
0.1
0.01
0.001
0.0001
availability limited
solubility limited
Total Content
matrix mineralogy
re-mineralization
Potentially Leachable
complexation (e.g., CI
-
, DOC)
solution
chemistry
CEMENT
MATRIX
neutralization
adsorption
2 4 6 8 10 12 14
pH
FIGURE 10.6
Factors controlling equilibrium-based leaching of metal constituents in dif-
ferent environments (modified from literature
10
).
10000
0.01
Available Content
1000
0.001
100
0.0001
10
1
0.00001
0 20 40 60 80
0 20 40 60 80
Time [days]
Mean Time [days]
a)
b)
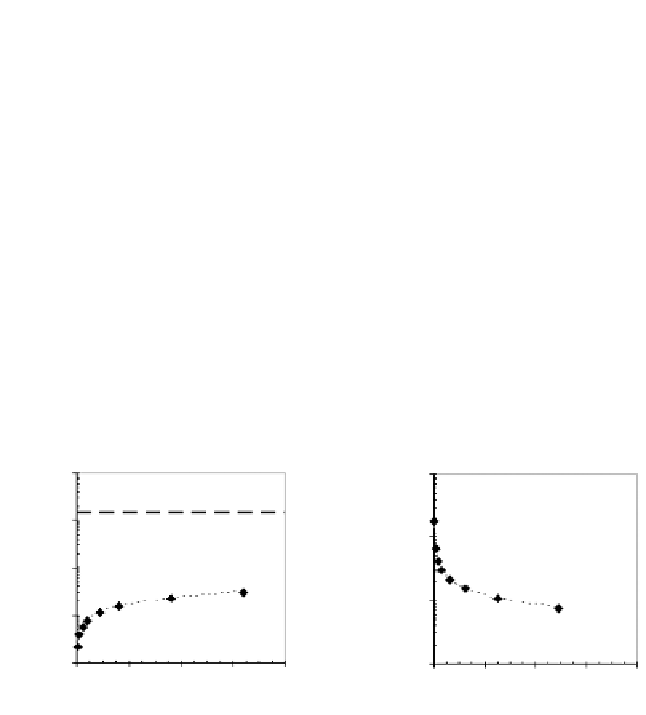
FIGURE 10.7
Schematic data from mass-transport rate tests presented as a) constituent
cumulative release [mg/m
2
] and b) constituent flux [mg/m
2
s].
designed to study the physical rate-limiting mechanisms of release. As such, a
maximum driving force for diffusion with no significant buildup of leachate con-
centration is assumed. This assumption may be valid only if leachate concentrations
are much less than the solubility concentration at the leachate pH (Figure 10.8b).
All mass-transport test concentrations in Figure 10.8b are below solubility limits.
However, the concentration in Leachate #2 is only slightly different from the solu-
bility concentration, indicating a potentially reduced driving force for leaching
during the second leaching interval.
10.5.2.1
Diffusivity in Reactive Media
Typically, the constituent diffusion rate through a matrix is characterized by a
diffusivity value; however, this approach is only valid for a limited number of cases
for which (i) the observed diffusion coefficient is constant in space and time, (ii)
the constituent of concern is not pH-dependent or no large pH gradients exist,

Search WWH ::

Custom Search