Environmental Engineering Reference
In-Depth Information
Fig. 2.14 The reacted
pathological detonation in p-
ʻ
plane
velocity. The pathological detonation discussed above is one example of eigenvalue
detonation [
10
13
].
Although the pathological detonation stated by Von Neumann is very interesting
in theory, its application value in condensed explosives is not clear yet. The time of
detonation reaction and positive pressure action of liquid explosives is far longer
than that of the condensed explosives. This explained why the detonation theory of
condensed explosives is not supported by the detonation theory of liquid explosives
[
14
-
16
].
This topic will discuss the detonation of liquid explosives
-
first exothermic
reaction, and the second exothermic reaction and endothermic detonation, which is
one example of eigenvalue detonation. This case has application value because
most of solid explosives are composed by one kind of explosives and a mixture,
which deactivates adhesives. In the detonation, the deactivation of adhesives is
endothermic. The eigenvalue detonation of this kind of explosives is achievable.
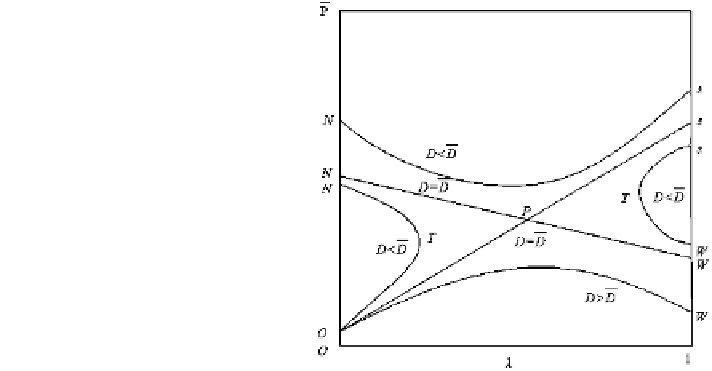
Figure
2.15
explains the key features of the eigenvalue detonation.
In the second stage after detonation initiation, there are two irreversible reac-
tions. The first is exothermic, and the second is endothermic. In the partial reaction
Hugoniot curve of p-V plane, Rayleigh line represents momentum and mass con-
servation rules. D is the eigenvalue detonation velocity/speed/rate. Only when
D ¼ D, under-pressure detonation point below point P is achievable.
—
the
2.2.2.3 Under-Pressure Detonation Wave of Liquid Explosives
in Pistons
The piston is introduced to discuss the detonation of liquid explosives [
17
].
Compared to the key features of condensed explosives, the piston issues/cases of
liquid explosives are a little more complex. Based on different piston rates
l
p
have