Environmental Engineering Reference
In-Depth Information
C, for each diethyleneglycol dinitrate molecule, the possibility of
obtaining evaporation energy is 1,012 times of activation energy. The evaporation
M
0
t
e
h
t=
RT
is much faster than the reaction MAe
E
=
RT
, even when M is larger than
M
0
. For some explosives, A is larger than
At 200
°
. For nitroglycerine, A = 1,020 and E is
also a large number (E = 179,912 J/mol). The speeds of evaporation and activation
still follow the above conditions. It is concluded that the transferred heat evaporates
the liquid molecules. The evaporation is much faster than reaction in the liquids.
Evaporation processes absorb both the transferred energy and the released energy
from reactions. The schematics of evaporation
υ
combustion are shown in Fig.
2.2
.
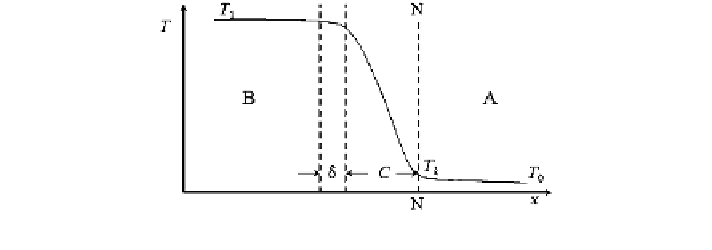
The temperature at the intersurface of the gas-condensed phase (N
-
N) is the
boiling point, at which the vapor pressure of liquid explosives equals the envi-
ronment pressure. The temperature of the condensed phase decreases with the
increase of the distance to the intersurface, and it gradually reaches the starting
temperature T
0
. Under this condition, there is a heating layer between gas and
condensed phases. When no evaporation occurs, the heating layer becomes thicker
with time prolong. In fact, because evaporation continues, the surface of condensed
phase shifts and the shift of stable combustion is much faster than the heat con-
duction heating condensed phase. As a result, the thickness of heating layer does
not change. According to some nonsystematic data, the heating layer of condensed
phase is several millimeters at most when the amount of explosive is not large and it
combusts. Because of the continuous evaporation of liquids, the preheating depth
(T
k
T
0
step) does not change. The vapor formed by evaporation of liquids does
not combust immediately; it needs time to be heated and for the reaction to pro-
gress. The combustion of vapor does not occur on the surface of the liquid, but at a
certain distance from the liquid. Combustion proceeds in the preheating zone. The
highest temperature of products is
-
Q
C
T
1
¼ T
0
þ
ð
2
:
5
Þ
Here, T
0
is the original temperature; Q is the energy released from the com-
bustion; and C is the heat capacity of products.
Later, the products are cooled down, and the temperature drops.
Fig. 2.2 Schematics of evaporation
combustion of
liquid explosives A condensed phase;
-