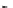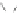Environmental Engineering Reference
In-Depth Information
(2) 2-chloro-1,3-propandiol as raw materials
Nitrificati
o
n
O
2
NOCH
2
CHCH
2
ONO
2
N
3
(II. MANG-1)
NaN
3
HOCH
2
CH
Cl
CH
2
OH
HOCH
2
CH
N
3
CH
2
OH
Hysrolysis
HAc
CH
NaN
3
OAc
CH
2
CH
2
OAc
AcOH
2
C
CHCH
2
OAc
N
3
Cl
(3) epoxy chloropropane as raw materials
NaN
3
CH
2
N
3
Ring opening with diluted
acid
CH
2
CH
O
HOH
2
C
CH
ONO
2
CH
2
N
3
H
2
C
CHCH
2
Cl
O
Nitrificatio
n
O
2
NO
CH
2
CH
ONO
2
CH
2
N
3
(III. MANG-2)
(1) Reaction mechanism of 1,3-diazido aminopropanol
NaN
3
DMF
ClCH
2
CHCH
2
Cl
OH
N
3
CH
2
CHCH
2
N
3
OH
1,3-dichloropropan-2-ol (76 g, 0.6 mol) and 150 mL DMF are added into a
500-mL three-neck bottle with stirring and thermometer. NaN
3
(108 g, 1.66 mol) is
added under stirring. The mixture is heated to 95
C and react for 90 min, then
cooled down to room temperature. CH
2
Cl
2
is used to extract for three times. The
extraction liquids are combined and washed three times. The separated organic
layer is dried using MgSO
4
. After
±
2
°
finally
1,3-diazido propan-2-ol (66.06 g) is obtained. The yield is 82 % with purity 99.2 %.
(2) Preparation of 1,3-azido-2-nitripropane
CH
2
Cl
2
(200 mL) and 1,3-dichloropropan-2-ol (46 g, 0.112 mol) are added into
a 1,000 mL three-neck bottle, and cooled down to 0
filtration, solvent CH
2
Cl
2
is evaporated;
°
C. HNO
3
(38 mL) is added
drop by drop in about 25 min. After 150 min reaction at 0
°
C, H
2
O is added and
stirred 15 min to separate the organic layer, which is washed 4 times using water.
After drying by MgSO
4
and
-
5
final product 1,3-
azido-2-nitripropane (16.68 g, 0.087 mol) is obtained and the yield is 79.46 %.
Nitri
filtration, CH
2
Cl
2
is evaporated. The
C produces nitroglycerine,
which is not the target product. Following the reaction temperature increase, the
purity of NG rises.
Experiments (Table
6.11
) indicate that if nitri
cation of 1,3-azido-2-nitripropane at 15
75
°
-
cation is conducted at high tem-
perature, azido group (
ONO
2
) gradually. The target
product is produced only under proper temperature range (
N
3
) turns to nitrate group (
-
-
5to5
°
C). Experiments
−