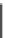Environmental Engineering Reference
In-Depth Information
Glycerine
5
H
2
O
0
CaCO
3
solution
ºC
ºC
68% nitric acid
cooling down 5
Added into the
reactor
Neutra-
lization
Ammonium
solution
Stirring
Stand
ºC
Quality
control
Quality
control
Dried
product
Washing
separation
Oil
extraction
NaCl
solution
Glycerine dinitrate
Fig. 5.12
The production process of glycerine dinitrate
Table 5.23
Consumption of raw materials for 100 kg glycerine dinitrate
Raw materials
Consumption (kg)
Glycerine
70.4
Nitric acid
240
Ice
80
Calcium carbonate
120
Ammonium sulfate
165
Ammonium
1.5
H
2
O
3,500
NaCl
5
Table 5.24
The fraction of nitroglycerine in glycerine dinitrate
Nitric acid
ratio
Products (kg)
Fraction (%)
Overall
esters
Glycerine
dinitrate
Nitoglycerine
Glycerine
dinitrate
Nitoglycerine
100:250
115.8
108.2
7.6
93.44
6.56
100:350
151.7
143.5
8.2
94.59
5.41
100:450
179.5
150.4
29.1
88.35
11.65
100:500
184.4
146.9
37.5
79.66
20.34
solution to be neutral. Figure
5.12
shows the production process and the con-
sumption of raw materials for producing 100 kg glycerine dinitrate is listed in
Table
5.23
.
The side products in the reaction include 200 kg ammonium nitrate and 165 kg
calcium sulfate.
The cost of production of glycerine dinitrate by this method is slightly lower
than the method of nitroglycerine as a raw material (Table
5.24
).