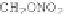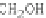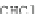Environmental Engineering Reference
In-Depth Information
temperature of distillation head is also controlled in a range. When the vapor
temperature is increased to about 100
ux in the condenser will be
observed. Two liquid layers will be formed in the segregator by further heating the
reactor. The supernatant is an ester layer and the subnatant is a water layer. The
water layer will be continuously separated out of the segregator and measured.
When the vapor temperature in the reactor is raised to 150
°
C, water re
fl
C and 75 % water is
separated by the segregator, the reaction will be ceased. It can be seen that the
supernatant in the reactor is light yellow, suggesting that esters are in the layer. The
subnatant is water. Cool down the temperature under agitation in air. At around
30
°
°
C in the reactor, liquid will be poured out and solid precipitates will be
filtered
away. There is obvious strati
filtrate. The target product of n-amyl
nitrate is in the supernatant and will be obtained after washing and drying. The
procedure of separation of waste acid from n-amyl nitrate is to wash the product
cation in the
five times the volume of n-amyl nitrate. The ester layer
after water washing will be washed by sodium carbonate
first by distilled water with
sodium chloride saturated
solution with pH 10 to alkalescence. The obtained liquid will be then washed by
sodium chloride saturated aqueous solution to pH 7.2
-
7.5 and magnesium sulfate
will be added for drying. The crude n-amyl nitrate is obtained with yield of 97.0 %.
The pure n-amyl nitrate with the yield of 92.4 % can be obtained by rectifying the
crude product with zeolite and cutting the fraction between 154.5 and 155.5
-
°
C.
5.2.7 Property and Preparation of Chlorinated Glycerol
Nitrate
Chlorinated glycerol nitrate is a homologue of nitroglycerine, in which a hydroxide
group is substituted by nitrate group and a hydrogen atom at carbon site of
methylene is substituted by a chloro group. Chlorinated glycerol nitrate is a nitrate
ester with very weak explosion property.
Molecular formula: C
3
H
6
NO
4
Cl
Chemical structure (three isomers with different substitution sites):
Molecular weight: 155.58
Oxygen balance:
51.42 % (CO
2
-based)
Nitrogen content: 9.00 %
Chlorinated glycerol nitrate is one of the liquid explosives with weak shock
sensitivity. It exploded only under the action of booster charges.
−