Environmental Engineering Reference
In-Depth Information
Fig. 4.6 Electrochemical
synthesis route of KDNE
K
NO
2
+KOH
NO
2
NO
2
+H
2
O
K
Fe
3+
Fe
2+
NO
2
NO
2
KNO
2
NO
2
K
K
NO
2
Fe
2+
Fe
3+
NO
2
NO
2
K
NO
2
K
NO
2
conditions with the combination of electrochemistry and crystallization process to
produce KDNE cleanly. It was also con
rmed that the electrochemical production
of KDNE can be achieved kg/h scale, and it is easy to transform DNPOH into
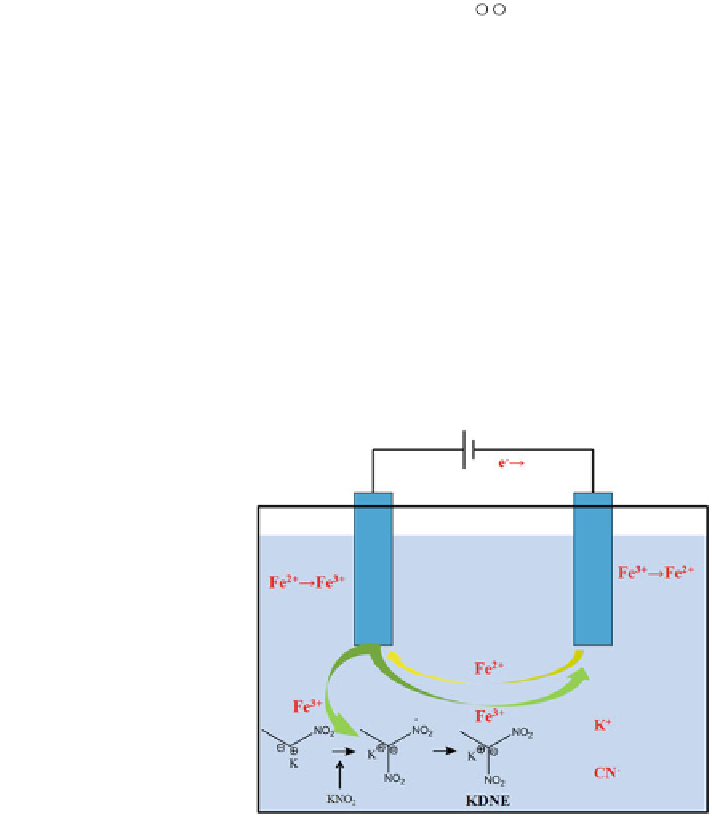
KDNE with existing equipments. Electrochemical synthesis KDNE is shown
schematically in Fig.
4.7
.
After the electrochemical production of KDNE, it is very easy to convert KDNE
into DNPOH on the industrial equipments. The conversion equipment is shown in
Fig.
4.8
.
In 2002, the cost of raw materials to synthesize DNPOH at ATK with traditional
production method ferricyanide was $17.5 per 1,000 g of BDNPF/A, while it is
only $8.34 with electrochemical synthesis route, which can be signi
cant cost
savings.
Fig. 4.7 Scheme of KDNE
electrochemical synthesis