Biology Reference
In-Depth Information
3
Methods
3.1 Cultivation
of Cell Suspensions
Perform all steps under sterile conditions in laminar fl ow box.
1. Day 0: inoculate 1 ml of BY-2 or LE cell suspension (station-
ary phase) into 30 ml of modifi ed MS medium in sterile 100 ml
Erlenmeyer fl ask. Alternatively, inoculate 2 ml of BY-2 suspen-
sion into 100 ml of modifi ed MS medium in 250 ml Erlenmeyer
fl ask. Inoculate 16 ml of VBI-0 cell suspension (stationary
phase) into 100 ml of V4 medium in 250 ml Erlenmeyer fl ask.
For cell suspension transfer, use cut off pipette tips. Close the
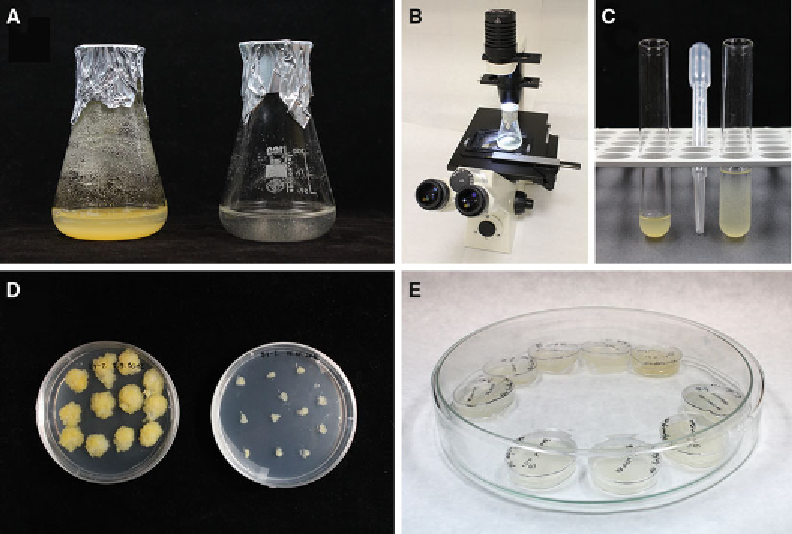
fl asks with aluminium foil (Fig.
1a
,
see
Notes 5
and
6
).
2. Cultivate the cells under continuous shaking on an orbital
incubator (orbital diameter 30 mm) in darkness at 27 °C and
150 rpm (BY-2 and VBI-0) or 25 °C and 130 rpm (LE).
3. Subculture into the fresh medium every 7 days (BY-2 and LE)
or every 14 days (VBI-0). During the SBI (subculture inter-
val), you may check the condition of cultured cells with inverted
microscope without opening the Erlenmeyer fl ask (Fig.
1b
).
Fig. 1
Equipment used for cultivation of and handling with suspension-cultured cells and calli. (
a
) 100 ml Erlenmeyer
fl asks with 7-day-old, stationary LE culture (
left
) and with subcultured aliquot (
right
). (
b
) Inverted microscope with
unopened (sterile) Erlenmeyer fl ask for direct observation. (
c
) Petri dishes (90 mm) with 5-week-old BY-2 calli (
left
)
and subcultured calli (
right
). (
d
) Sterile glass Petri dish with 60 mm Petri dishes after transformation. (
e
) Rack with
test tubes with LE cells undiluted (
left
) and diluted (5×), prepared for cell density counting
Search WWH ::

Custom Search