Biology Reference
In-Depth Information
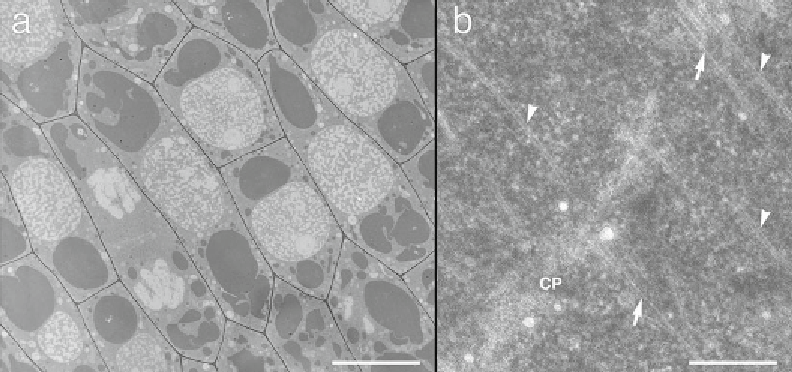
Fig. 2
Tangential longitudinal sections of high-pressure frozen onion epidermal cells embedded in Spurr's
resin. (
a
) A lower magnifi cation view. Scale bar: 10
m. (
b
) A higher magnifi cation view showing a portion of a
developing cell plate (CP). Microfi laments (
arrows
) are clearly seen, as are microtubules (
arrowheads
). Scale
bar: 0.25
μ
μ
m
5. Using a steak knife tip or other sharp tool, remove kernels by
cutting through the cob under the kernel. Place the cutting
tool far enough away from the kernel so that the basal part of
the kernel is not damaged.
6. Samples should look yellowish in 100 % HM20 resin because
the FS medium contained UA.
7. For conventional electron microscopy, prepare formvar fi lm
with a thickness of ~80 nm. The fi lm thickness can be esti-
mated from its interference color; the color of an ~80 nm fi lm
is silver. For electron tomography semi-thin sections (e.g.,
250 nm or more) are used, and thus the interference color of
the formvar fi lm should be dark gold, because the formvar fi lm
can be easily broken if thinner. It is convenient to use a glass
slide lifter (MA-1, Nisshin EM, Tokyo, Japan) for formvar
coating, if available. Electron micrographs of ultrathin sec-
tions of typical well-frozen onion samples are shown in Fig.
2
.
Acknowledgments
This work was supported by JSPS KAKENHI Grant (No. 24620003)
to I. K. and NSF (No. MCB 0958107) and USDA (AFRI 2010-
04196) to B.-H. K. We are thankful to Dr. Mineyuki (University of
Hyogo) and Donna S. Williams (University of Florida) for their
careful reading and comments.
Search WWH ::

Custom Search