Agriculture Reference
In-Depth Information
+
NH
4
OH
-
(1)
+
+
H
2
O
NH
3
Ammonium ion
H
+
+
H
3
O
+
H
2
O
(2)
Hydronium ion
H
H
NH
+
H
H
O
O
H
H
+
O
H
O
NH
+
H
H
H
+
H
O
H
O
O
NH
+
H
H
O
H
H
O
O
NH
+
M
+
H
+
H
H
O
O
H
H
3
O
+
O
NH
+
H
NH
+
Humus
NH
+
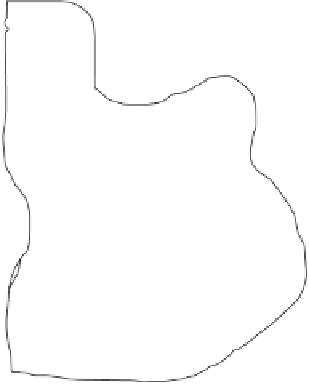
Figure 4.6.
Reactions forming ammonium and hydronium ions. Ammonium ions associated with
exchange sites on soil humus.
negative oxygens with cations, forming a “shell” of water molecules around
them as illustrated in Figure 4.6. Most cations in soil are simple metal ions,
although some, such as iron, may be present in multiple oxidation states. An
exception to this is molybdenum, which occurs not as a cation but as molyb-
date, an oxyanion. Two nonmetal cations in soil are ammonium and hydronium
(hydrogen ions, protons, associated with water). In contrast there are relatively
few simple anions found in soil out side of the halogens, particularly chloride,
Cl
-
, and bromide, Br
-
. Most anions in soil occur as complex oxyanions.
4.7.1.
Simple and Multi-Oxidation-State Cations
The most common simple cations in the soil solution are calcium (Ca
2+
), mag-
nesium (Mg
2+
), potassium (K
+
), and sodium (Na
+
). Other alkali and alkaline-
earth elements, when present, will be as simple cations also. Iron, aluminum,
copper, zinc, cobalt, manganese, and nickel are also common in soil. Iron is
present in both the ferrous (Fe
2+
) and ferric (Fe
3+
) states, while aluminum will
be present as (Al
3+
). Copper, zinc, cobalt, and nickel can all be present in one