Agriculture Reference
In-Depth Information
B
A
B
A
P
P
cP
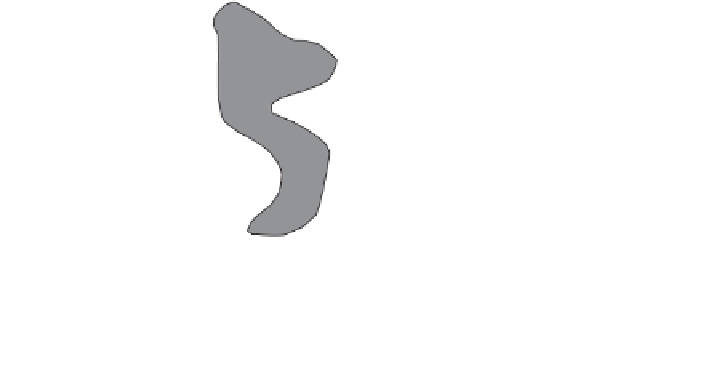
Figure 4.4.
Two peds (
AA
and
BB
) each have a pore that does not drain because the mouths are
too small. Ped
BB
has a pore closed at one end (i.e., cP). Between the peds is an apparent pore
(aP) formed by the close proximity of the peds.
4.3.
SOLUBILITY
The solubility of components in the soil solution will be controlled by the
innate solubility of the compound in question and the existing soil solution
characteristics, particularly salts already present. High salt concentrations will
result in salting out and precipitation of some components. Note here that salt
concentration is not constant because as the soil dries, the concentration of
salt increases and precipitation reactions that may not be reversible when the
soil water content is subsequently increased.
In addition to straightforward precipitation reactions, components may dis-
solve and react with components already present, including atoms on colloidal
surfaces. For example, phosphate may dissolve from phosphate rock and react
with iron present in the soil solution or on particle surfaces to form an iron
phosphate that is insoluble.
4.4.
ELEMENTS IN SOLUTION
Elements in the soil solution will be derived from the atmosphere, lithosphere,
and biosphere. Thus nitrogen, oxygen, and argon from the atmosphere will
commonly be found dissolved in soil water. It is often surprising and some-
times disconcerting to find argon in soil air and water primarily because it is
rarely mentioned as a component of air. Although its occurrence may be sur-
prising, it does not represent an unusual situation.
Mercury and the noble metals are found in nature in their elemental forms;
however, they are generally unreactive and so their occurrence in the soil solu-