Agriculture Reference
In-Depth Information
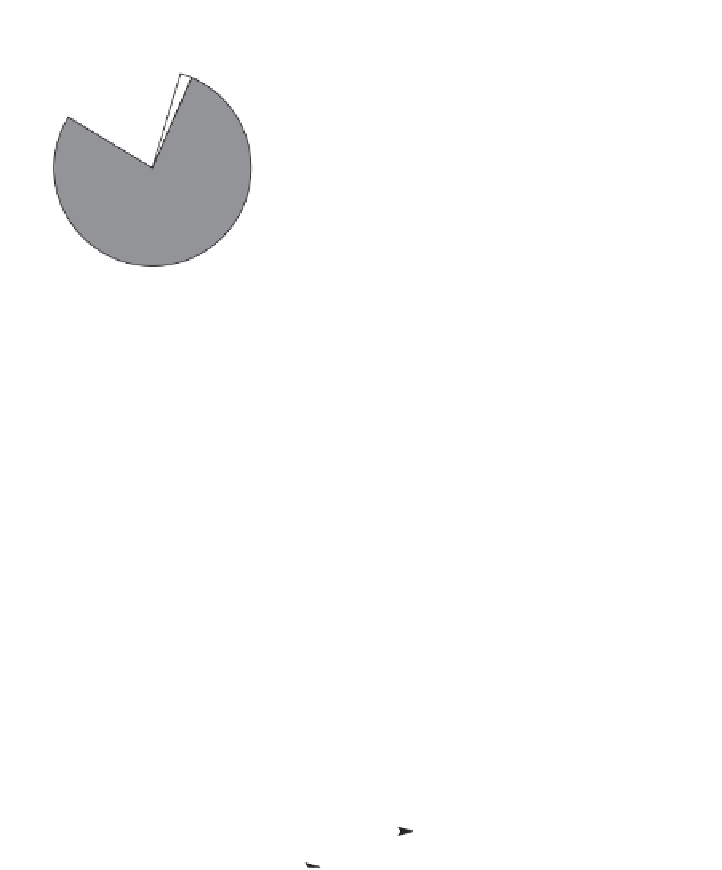
Soil atmosphere
Nitrogen
Oxygen
Argon and
Carbon dioxide
Other
Aerobic
Anaerobic
Figure 4.1.
Soil atmosphere composition under aerobic and anaerobic conditions.
oxidation reactions predominate. When the void volume is occupied by water,
the soil is anaerobic and reducing reactions predominate.
Oxidation reactions in soil, particularly those carried out by microorgan-
isms, and plant roots increase the amount of carbon dioxide in soil air to 10
or more times the concentration in atmospheric air. The consequence of this
is that the oxygen content is proportionally decreased. When the soil void
volume is almost or completely filled with water, the remaining trapped and
dissolved oxygen is quickly utilized by organisms and the oxygen content of
any remaining gas is zero. The soil is then anaerobic and reducing conditions
prevail (see Figure 4.1).
In addition to oxidation-reduction reactions occurring under various con-
ditions, increased carbon dioxide will also affect soil pH. Carbon dioxide dis-
solves in water, producing both bicarbonate and carbonate, which will release
protons into the soil solution as shown in the following reactions, which illus-
trate the involvement of CO
2
in control of soil water pH:
H
2
O + CO
2
H
2
CO
3
(4.1a)
H
+
+ HCO
-
(p
K
1
6.38)
(4.1b)
H
2
CO
3
HCO
-
H
+
+ CO
2-
(4.1c)
(p
K
2
10.25)
H
+
+ CO
2-
HCO
-
(4.1d)
Ca
2+
+ CO
2-
CaCO
3
(4.1e)
In addition to providing protons, carbonic acid can react with cations to form
insoluble precipitates. Both reactions can dramatically alter the composition
of the soil solution and soil extracts.
Other gases in the soil atmosphere also change, and thus so will the gaseous
components dissolved in soil water, causing its composition to change.