Agriculture Reference
In-Depth Information
S
2
O
2-
SO
2-
SO
2-
Oxidation
R-OSO
3
H
S
0
R-SO
3
H
R-S-R
H
2
S
R-SH
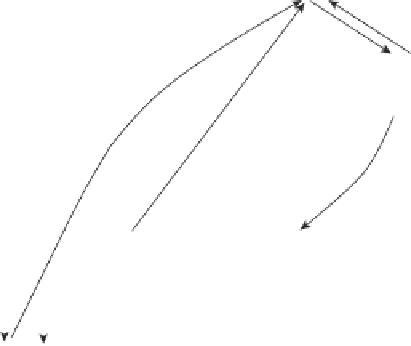
Figure 3.8.
The sulfur cycle where S
0
is elemental sulfur, H
2
S is hydrogen sulfide, S
2
O
2-
is thio-
sulfate, SO
2-
is sulfite, SO
2-
is sulfate, ROSO
3
H represents a sulfate ester, RSO
3
H a sulfonic acid,
RSR a thioether, and RSH a thiol (adapted from Coyne MS.
Soil Microbiology: An Experimen-
tal Approach.
Boston: Delmar Publishers, 1999).
BIOLOGICAL AND ORGANIC CHEMICALS OF SOIL
3.4.
BIOCHEMICAL
There are two sources of biochemicals in soil; one is the cellular constituents
released when cells are destroyed during the extracting process. It should be
kept in mind that any handling of a soil sample will cause the destruction of
some of the cells it contains. The simple acts of sieving, air drying, and weight-
ing soil will cause some lyses of cells and release of their contents. Extraction
will typically cause complete destruction of all cells in soil, with the release of
all their constituent parts. Some parts such as enzymes may continue to func-
tion after release from the cell and continue to change the makeup of soil com-
ponents for some time.
The second source of biochemicals is molecules excreted from cells such as
extracellular enzymes and unused organic matter. A typical example is cellu-
lase, which is excreted by fungi such as
Penicillium
in order to break down
wood and woody material into sugars that can be used by the organisms.
Other common extracellular enzymes found in soil are ureases and amalyases.
Often enzymes are associated with clay particles, and in such associations their