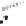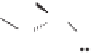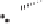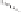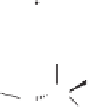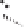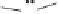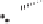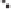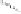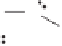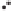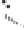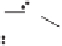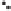Agriculture Reference
In-Depth Information
O
O
O
O
O
O
O
O
Al
O
Si
O
O
Si
O
O
O
HO
HO
OH
OH
HO
OH
OH
Al
Al
Mg
Al
O
O
O
O
O
O
O
O
O
O
O
O
Si
Si
Si
O
O
Si
O
O
O
O
O
O
O
O
O
O
H
H
H
H
O
O
O
O
H
H
H
H
O
O
O
O
O
O
O
O
Si
O
Si
O
O
O
Si
O
O
Si
O
O
HO
HO
HO
OH
OH
OH
OH
Al
Al
Al
Al
O
O
O
O
O
O
O
O
O
O
O
Si
Si
Si
O
O
Si
O
O
O
O
O
O
O
O
O
O
n
n
n
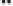
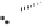
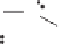
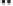
Figure 2.3.
Two types of isomorphous substitution. The middle structure is a two-dimensional rep-
resentations of clay without isomorphous substitution. On the left is isomorphous substitution of
Mg for Al in the aluminum octahedral sheet. On the right is isomorphous Al substitution for Si
in the silicon tetrahedral sheet. Clays are three-dimensional, and —OH on the surface may be
protonated or deprotonated depending on the pH of the surrounding soil solution. There will be
additional water molecules and ions between many clay structures. Note that clay structures are
three-dimensional and these representations are not intended to accurately represent the three-
dimensional nature or the actual bond lengths; also, the brackets are not intended to represent
crystal unit cells.
have substitution in the aluminum octahedral sheet. For instance, an octahe-
dral sheet might have substitution of magnesium for aluminum. These two sub-
stitutions were chosen to illustrate that with substitution some bonds in the
clay structure will go unsatisfied. This means that some bonding electrons will
not be shared between two atoms, resulting in the clay having a negative
charge that is satisfied by the same cations discussed for kaolinite (Section
2.1.3.1). This results in cation exchange capacity greater than that ascribed to
edge effects alone.
The Fine Grained Micas.
The fine grained micas are distinguished by
having 2 : 1 structure and are nonexpanding when the water content of their
surroundings changes. Isomorphous substitution is in the silica tetrahedral
micas, and causes a change in the shape of the crystal. Thus this portion of