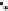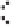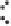Agriculture Reference
In-Depth Information
O
OH
OH
O
O
O
O
O
Si
Al
Al
O
O
O
O
HO
OH
OH
OH
d
-
O
d
+
d
+
HH
Figure 2.1.
A silicon tetrahedron (left), an aluminum octahedron as a central layer in a 2 : 1 clay
and an aluminum octrahedron as a surface layer in a 1 : 1 clay (right). Both the oxygen and OH
groups are bonded to other silicon and aluminum atoms in the clay (bonds are not intended to
be shown at the correct angels). Below is a water molecule showing partially positive hydrogens
and partially negative oxygens. Also shown are the two lone pairs of electrons on all the oxygens.
oxygen and hydroxy groups bonded to silicon determines the chemical reac-
tivity of freshly formed surfaces of this soil fraction.
Quantifying silica interactions with its surroundings is difficult. First, the
surfaces are not regular, and thus it is impossible to calculate their area.
Surface areas must be measured, and although surface area measurement is
not difficult, it is time-consuming and open to inaccuracies. Second, as noted
above, the surfaces are irregularly covered and it is impossible to know the
extent, type, and thickness of materials covering all the surfaces. However,
silica bonds and electron pairs are important in any chemical analysis, analyt-
ical procedure or instrumental procedure applied to soil.
2.1.2.
Silt
The silt fraction is particles 0.02-0.002 mm in diameter. This fraction or sepa-
rate is produced by the same physical processes as described above for the
formation of sand. Silt is more finely divided silica, but the surfaces are basi-
cally the same as those of sand (i.e., silicon), and oxygen lone pairs of elec-
trons and hydroxy groups control its chemistry. Because the particles are
smaller, they have a larger surface area, that is, more surface per unit mass.
This results in the availability of a greater number of bonds for chemical reac-
tions. However, again, although the amount of surface area can be measured,
the availability of silicon, oxygen lone pairs of electrons, and hydroxy groups
for chemical reaction cannot be known exactly [1].
2.1.3.
Clay
The next smaller separate is actually a group of particles of differing types col-
lectively called
clay
and are particles measuring less than 0.002 mm in diame-
ter. They are significantly different from sand and silt separates both physically