Agriculture Reference
In-Depth Information
aby
Mobile
phase
Stationary
phase
b b
aby
b
a y
Mobile
phase
a a
a y
b
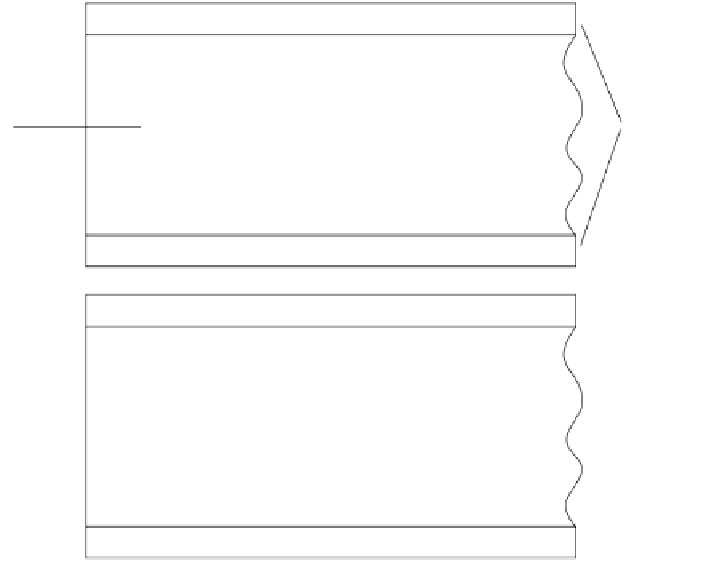
Figure 9.1.
Partitioning of the components of a mixture in a chromatographic column.
This partitioning-separation process is diagramed in Figures 9.1 and 9.2. In
Figure 9.1 a mixture is introduced into a chromatographic column. The com-
ponents are adsorbed on the stationary phase, and the component more
soluble in the mobile phase moves into this phase and is swept along the
column, where it is adsorbed again. The less soluble component then moves
into the mobile phase and is again readsorbed by the stationary phase. In
Figure 9.2 a mixture is introduced into a chromatographic column, and as
its components move down the column, they are separated into discrete
“packets” of the same compound and on exiting the column, they are detected.
Table 9.1 summarizes the common chromatographic methods and the char-
acteristics that differentiate them from other methods. In this list HPLC and
GC are most commonly used in analyzing soil extracts, particularly when they
are used in combination with mass spectral methods.
9.2.
GAS CHROMATOGRAPHY
Gas chromatography is a powerful, rapid method for separation of mixtures
of gases and compounds with boiling points below
~
400°C.
2
The sample, when
2
The temperature value 400°C is not an absolute upper limit of GC but is near the upper limit
of most GC columns.