Agriculture Reference
In-Depth Information
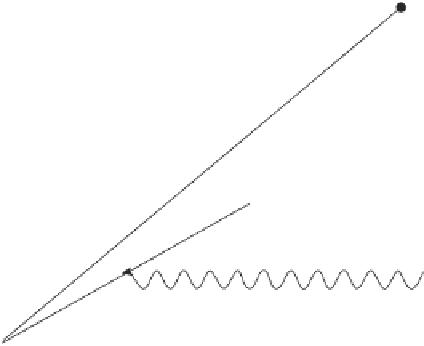
Ejected
electron
Incoming
X-ray
Electron falling to
lower level
Fluorescence
photon
Figure 8.3.
Diagram showing the source of X-ray fluorescence photons.
often a metal, will, in part, determine the range of elements that can be
detected.
When used in direct soil analysis, X-ray fluorescence suffers from the fact
that it is largely a surface phenomenon. For this reason, only surface elements
will be determined. However, because it is a surface phenomenon, it has been
extensively used to study sorption on the surfaces of soil components. An
extensive list of investigations using XRF to investigate various sorption
mechanisms is given in the text by Sparks [6].
Other characteristics of XRF that can limit its usefulness are the surface
area observed and surface contamination. In XRF the surface area measured
is small, meaning that a large number of determinations must be made in order
to obtain a representative sample of the elements present. In addition, trans-
port and storage of uncovered soil samples can lead to surface contamination
that will subsequently appear as part of the soil constituents.
The sensitivity of X-ray fluorescence determinations is better for elements
with higher mass number and less for lighter elements. Often in soil analysis
lighter elements are of greater interest, and this makes application of this
method more difficult. Typically determination of elemental composition in
the part per million (ppm) range is, however, achievable.
Fluorescence determinations are best made on samples of homogeneous
particle size, and this is not the normal state of soil. Grinding and carefully
sieving soil before analysis can minimize problems associated with particle size
heterogeneity. Another approach has been to fuse soil with borate or dilute it