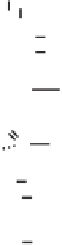Agriculture Reference
In-Depth Information
H
3
O
+
s
H
3
O
+
e
H
b
Ca
++
+
H
3
O
e
+
H
3
O
e
H
b
+
H
3
O
s
+
O
C
H
b
+
H
3
O
s
Na
+
Na
+
H
3
O
e
+
Figure 6.4.
An equation showing the equilibrium between bonded protons (H
b
), exchangeable
protons (H
3
O
e
), and soluble protons (H
3
O
s
) is given above and illustrated below.
between H
3
O
+
in solution and on exchange sites (Figure 6.4). In addition there
may be a release of weakly held protons from either or both inorganic or
organic constituents in soil. It might be envisioned that there is an equilibrium
between all three sources of protons and that the decrease is a return to
reestablishing this equilibrium.
In Figure 6.4 these three sources of protons are illustrated and designated
as H
3
O
s
hydronium ions in solution, H
3
O
e
hydronium ions on exchange sites
and H
b
protons bonded to some soil constituent by either a covalent or polar
covalent sigma bond. As discussed in previous chapters, measuring soil pH
using a salt solution results in a lower pH being found. Here the cation pro-
vided by the salt replaces protons or hydronium ions on exchange sites, and
thus they are in solution and can be measured. When a base such as NaOH is
added to soil, the Na
+
cation will exchange with protons or hydronium ions on
exchange sites in a similar manner. In addition, every proton exposed to the
soil solution will have a p
K
a
value and thus be released or bonded depending
on the pH of the solution. What is seen is that as the solution is made, more
basic protons from all these various sources are potentially released into
solution.
If a slow continuous addition of base is made to the same soil used in Figure
6.3, a similar titration curve without the sawtooth pattern is seen. Figure 6.5
shows the titration curve obtained by the continuous slow addition of 0.1 M
NaOH. Again the curve is not a smooth line, and irregularities found in this
titration are seen in other titrations of this same soil. Note that no distinct