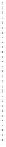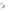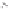Agriculture Reference
In-Depth Information
300 mv
Salt bridge
KCl Cl
-
K
+
Glass wool plug
Zn
2+
Cu
2+
SO
4
2-
SO
4
2-
ZnSO
4
CuSO
4
Figure 5.2.
A typical electrochemical cell.
A basic electrochemical cell (depicted in Figure 5.2) consists of a copper wire
in one container with a solution of copper sulfate and a zinc rod in a differ-
ent container with a zinc sulfate solution. There is a salt “bridge” containing a
stationary saturated KCl solution between the two containers. Ions flow freely
in the salt bridge in order to maintain electrical neutrality. To complete the
cell, a wire is connected to each rod and then to a measuring device such as a
voltmeter.
5.2.
ELECTRICITY GENERATION IN SOIL
The generation of small electrical currents in soil is possible and may affect
any electrical measurement made therein. More recent theoretical and exper-
imental work has shown that charged particles, including ions, passing through
microchannels under low pressure (i.e., 30 cm of water), can generate small
amperages. In addition, amperages from multiple channels are additive and
might be a useful method of generating electricity or of making a new type of
battery [1,2].
Soil solids have channels having charged surfaces, such as those in clays,
and what is termed an
electrical double layer
on the surfaces and salts in the
soil solution moving over and through them. The generation of electricity in
soil by this mechanism is thus possible. Although generation of electrical cur-
rents under standard extraction conditions may not be of concern, they may
be in other soil analytical procedures. In situ measurements, taken with elec-
trodes buried in soil, may record or be susceptible to interference from such
currents. This will be particularly true for electrodes buried in fields, during