Environmental Engineering Reference
In-Depth Information
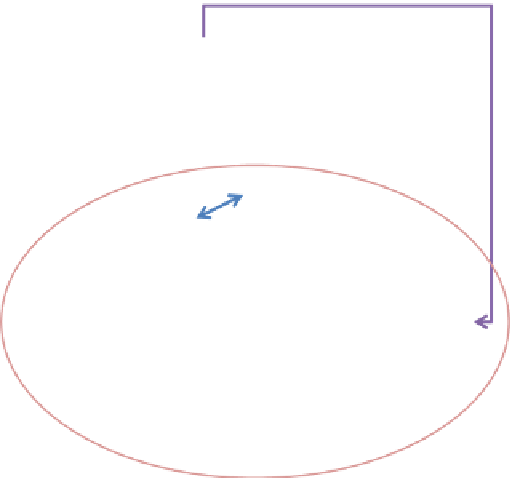
Human perturbations to atmospheric iron deposition to oceans
Human
emissions
CO
2
, SO
2
, NO
x
,
Fe
Human
perturbations
Human land
use
Desert Dust
Climate
Soluble iron
deposition to
oceans
CO
2
concentrations
(other GHG,
aerosols)
Natural cycle of how
desert dust iron may
modulate ocean co
2
uptake
Ocean
productivity
Fig. 2.2 Skematic representing feedbacks between natural ocean carbon cycle, carbon dioxide
concentrations, and iron inputs also shows humans could be perturbing the iron deposition
(Figure from [
65
])
estimated to be less than 5% of the total iron in aerosols [
64
,
65
]. Crustal material is
on average about 3.5% iron [
66
], with some minerals having substantially higher or
lower concentrations [
67
,
68
]. However, estimates and observations suggest that
desert dust aerosols vary in their iron content by only a factor of 2, suggesting that
the high heterogeneity in the soils is mixed in the atmosphere [
65
,
69
]. There are
also small sources of atmospheric iron from volcanoes [
70
,
71
], cosmic dust [
72
],
and combustion [
64
].
Because atmospherically deposited dust only resides in the mixed layer of the
ocean for a short time, many researchers consider the soluble fraction of the iron the
most relevant for ocean iota [
73
]. However, which fraction of the iron is really
bioavailable is not well understood, but is likely to be a small fraction of the total
iron in aerosols (1-80%) (see reviews in [
65
,
73
]). Because the soluble fraction of iron
in soils is much smaller than what is observed in the atmosphere, it is thought that
atmospheric processing of iron is important [
73
,
74
]. It is likely that the acidity and
insolation play a role in processing of iron [
73
-
75
]. Some iron-containing minerals
are more easily solubilized than others and iron in combustions is significantly more
soluble than iron in mineral aerosols; these factors complicate our understanding of
iron solubility [
68
,
76
-
78
](
Fig. 2.2
from [
65
]).
Some regions of the ocean are iron deficient and additions of iron can result in
phytoplankton blooms [
79
,
80
]; however,
there is limited evidence showing