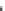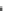Environmental Engineering Reference
In-Depth Information
Element Concentration (
m
M)
0
5
10
25
30
0
50
100
150
200
Dissolved Inorganic
Particulate
250
Fig. 12.1 Generalized
vertical
distributions of dissolved inorganic elements and particulate
matter produced by phytoplankton photosynthesis. Particulate matter concentrations are less
than those expected from the disappearance of inorganic elements because of removal by various
processes to depth (see
Fig. 12.2
). Similarly, the particulate matter
vertical
distribution is less
uniform because the time-scale of redistribution of particles is much faster than that of the
inorganic elements
(
$
) organic carbon-nitrogen-phosphorus compounds
ð
CH
2
O
Þ
106
ð
NH
3
Þ
16
H
3
PO
4
and gaseous oxygen (
O
2
). The numbers preceding the compounds indicate the amount
of each. The relationship is reversible (
) because metabolism (oxidation) of the
organic matter produced by photosynthesis regenerates inorganic C, nitrogen (N),
and phosphorus (P) in the same ratio and utilizes oxygen. The C:N:P ratio of 106:16:1
obtained from the above relationship is a basic paradigm of marine biogeochemistry.
However, Redfield recognized that the C:N:P ratios vary within plankton types and
with time, a fact that has been further established in more recent studies. The
departure from the basic ratio provides insights in how marine ecosystems change
and/or adapt to modified environmental or biological conditions.
All marine organisms contribute to the carbon cycle by moving carbon between
organic and inorganic forms, but some marine organisms are able to use calcifica-
tion to transform inorganic carbon, using bicarbonate and dissolved calcium from
the water column to produce calcium carbonate (CaCO
3
),whichisthenusedto
form a skeleton or protective shell [
2
]. The dissolution of calcium carbonate back
into its original components is one of the primary means by which the particulate
components reenter the water column, keeping the inorganic carbon cycle running.
Although some of the calcium carbonate dissolved back into the water column
comes from dead organisms, a large portion is contributed by phytoplankton from
$