Biology Reference
In-Depth Information
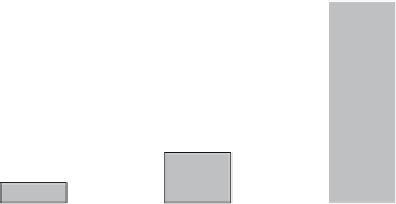
250
200
150
100
50
0
A
B
C
Fig. 3. Quantitation of lipid damage.
Borrelia burgdorferi
strain B31A3 was grown
to a cell density of 5 × 10
7
cells/mL in BSK II under anaerobic, microaerophilic,
or aerobic conditions (
A
,
B
and
C
, respectively) at 34 °C for 3-5 days. Cells were
harvested, lysed, and reacted with TBA. The resulting thiobarbituric acid reacting
substances (TBARS) are then injected onto a C18 HPLC column and the absorbance at
532 nm monitored. Standards made from pure MDA were used as controls. The data
is calculated as microliters MDA per 10
8
cells.
7. Add 2mL rabbit serum to stop the reaction. Incubate for 1min at room temperature.
8. Add 4 mL HEPES/NaCl buffer.
9. Centrifuge mixture 1000 ×
g
for 10 min at room temperature. Wash cells thrice
with HEPES/NaCl buffer.
10. Resuspend cells in 1 mL of HEPES/NaCl buffer and incubate at 34 °C for 5 min.
11. Add 30 μL of 2.5 m
M
DPPP.
12. Incubate for 5 min at 34 °C in the dark.
13. Centrifuge the mixture 1000 ×
g
for 10 min at room temperature. Wash pellet
thrice with HEPES/NaCl buffer.
14. Resuspend cells in 100 μL of HEPES/NaCl buffer.
15. Before microscopy, the cells must be fixed by adding paraformaldehyde to 2%.
16. Pipet 5 μL of fixed cells onto a microscope slide.
17. Add 5 μL of embedding solution. Cover with coverslip.
18. To visualize
a. Red flourescent cell linker, excitation = 551 nm and emission = 567 nm.
b. DPPP, excitation = 351 nm and emission = 380 nm.
3.5. Identification of Lipid Peroxidation Intermediates
1. Grow and treat cells as described above.
2. Centrifuge 10
10
cells at 3000 ×
g
for 15 min. Wash the cells thrice in HEPES/NaCl
buffer. Resuspend pellet in 50 μL HEPES/NaCl buffer.

Search WWH ::

Custom Search