Biology Reference
In-Depth Information
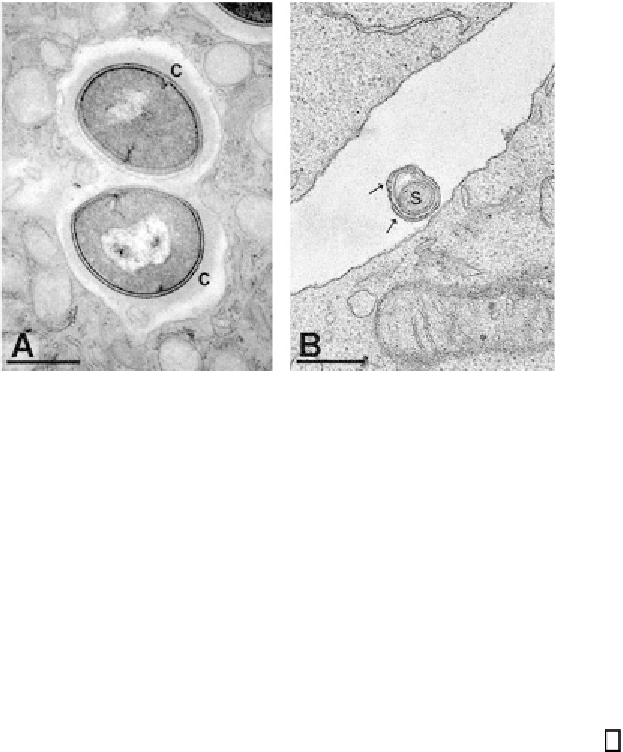
Fig. 1. Transmission electron micrographs showing sections of bacteria and host
cells.
(A)
Digital image of methicillin-resistant
Staphylococcus aureus
within a primary
human neutrophil. Bacterial capsular material (c) is preserved within the endosome.
Cytoplasmic vesicles are evident in association with the endosomal membrane.
(B)
Photographic image of
Borrelia burgdorferi
and lymphocytes. In cross-section, two
layers of extra membrane (arrows) are evident surrounding the spirochete (
s
) (unpub-
lished data). Bars, 500 nm.
for perfusion of tissues. As the rate of diffusion and thus fixation is dependent
on the biochemical nature of the material and physical properties such as
temperature and sample thickness, the length of time required is variable and
largely unpredictable. Organisms with thickened, resistant, and/or hydrophobic
cell walls such as Gram-positive bacteria, spores, or mycobacteria require
extended fixation or enhanced processing, such as microwave irradiation
(4)
or both. Experimental assessment of viability during fixation can be useful for
determining the necessary length of treatment. Membranes and storage lipids are
stabilized by cross-linking with oxidative osmium species. Osmium treatment
both reduces the quantity of lipids extracted during preparation and provides
contrast for lipophillic structures. Contrast is often enhanced by subsequent
staining with metallic salt solutions, such as uranyl acetate and lead citrate.
To enable thin sectioning, the samples are dehydrated in organic solvents
and infiltrated with epoxy or acrylic resins, which are then polymerized to
provide support and minimize compression artifacts during sectioning. As with
fixation steps, the duration period and processing techniques for dehydration
and embedment may require adjustment depending on types of samples and
viscosity of the resin.
Search WWH ::

Custom Search