Environmental Engineering Reference
In-Depth Information
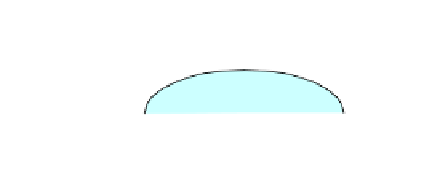
problem is formalized by Young's equation [9.6] that translates the mechanical
equilibrium of a liquid drop on a solid surface; see Figure 9.2.
α
σ
lg
σ
sg
liquid
σ
sl
solid
Figure 9.2.
Drop of water on a solid surface
σ
cos
α
=
σ
+
σ
[9.6]
lg
sg
sl
In the above equation, σ
lg
,
σ
sg
and σ
sl
are
surface tensions
(forces per unit
length: N.m
-1
) existing at the liquid/air, solid/air and solid/liquid interfaces,
respectively. α is the
wetting angle
that characterizes the wettability of the solid by
the liquid considered. Solving [9.6] can yield three main results:
- α < 90°: the liquid is
wetting
(α = 0: perfectly
wetting
);
-
α > 90°: the liquid is
non
-
wetting
. It does not spread naturally on the solid
surface;
- no solution: there is an unstable configuration.
Most minerals in stones have surface defaults in their crystalline network which
create surface charges that can interact with a dipolar solvent like water. This is why
water is generally perfectly wetting towards solid stone surfaces. Nevertheless,
treatment with hydrophobic agents, for example, or some surface weathering
patterns (colloid formation due to bacteriologic activity, lichens, patina, etc.) can
alter - or even invert - this behavior.
9.2.2.2.
Laplace's law
In the general case, water - as a wetting fluid on stone surfaces as discussed
above - has a tendency to spread over the whole surface of the inner stone's porous
structure, thus naturally penetrating inside. This phenomenon, which is
capillarity
,
reaches equilibrium through the formation of
meniscus
: water/air interfaces inside
the porous structure that is illustrated in Figure 9.3.