Biology Reference
In-Depth Information
Airway Lumen
BAL/sputum
apical expression
(CLCA1)
FeNO
IL5
IL13
Intracellular expression
(iNOS)
CCR3
chemokines
Basolateral expression
(periostin)
Eosinophil
Bronchial Tissue
Bloodstream
Serum/plasma
FIGURE 4.2
Relationships between type 2 cytokines, bronchial mucosa, and biomarkers. IL13 induces the
expression of genes encoding periostin, CLCA1, iNOS, and CCR3-binding chemokines (e.g., CCL13, CCL26) in
bronchial epithelial cells. IL5 induces eosinophil hematopoiesis and CCR3-binding chemokines recruit eosino-
phils to bronchial tissue. In bronchial epithelial cells, CLCA1 protein is expressed on the apical surface, iNOS is
expressed intracellularly, and periostin is secreted from the basolateral surface. Secreted periostin protein is detect-
able in peripheral blood, while exhaled nitric oxide (FeNO) is detectable in exhaled breath.
may be variably present in a given clinical sample. Polyclonal antibodies raised to full-length
periostin protein may contain mixtures of individual antibodies that bind to epitopes present in
only a subset of periostin splice variants. To develop a prototype clinical-grade assay for peri-
ostin, we selected monoclonal antibodies for both capture and detection that bound to epitopes
in the conserved N-terminal region of the known splice variants of periostin, so that the assay
provided a true estimate of total periostin protein in a clinical sample
[46]
.
Figure 4.3
depicts a
simplified theoretical example of how varying isoform mixtures in two clinical samples with
identical levels of total periostin protein could yield different outcomes with a polyclonal anti-
body (pAb)-based immunoassay. A more general challenge in developing regulated clinical
diagnostic tests is reproducibility of the assay over time across manufacturing lots. Production
of pAbs relies on affinity purification of antibodies from serum of immunized animals, which
is an exhaustible supply of reagent. Once the serum from a particular bleed is consumed, addi-
tional bleeds from the same animal taken at different times and/or serum from other immu-
nized animals must be used to produce additional pAbs, which are likely to consist of different
mixtures of individual antibodies over time, making assay standardization difficult. As mono-
clonal antibodies (mAbs) are produced from cell lines and consist of single purified clones, it is
substantially more feasible to ensure assay consistency over time across manufacturing lots.





















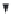


























Search WWH ::

Custom Search